Your Aging Immune System and COVID-19
Your aging immune system and COVID-19 don’t play well together. There’s a reason that those in their sixties and older are suffering from this virus more than younger folks. Find out why and what to do about it.
Check out my Covid Immunity CourseAs of May 2, 2020, more than a dozen states have reopened, thus ending the lock-down most have endured for several weeks. (Here’s a map of those opened and those still shut down.)
Now that many of us can tentatively leave our homes and enter society, more or less (mostly less), what’s on everybody’s mind is how to protect ourselves and each other from SARS-Cov2, the virus responsible for COVID-19. (COVID-19 refers to the disease caused by SARS-Cov2).
What we know is that, although the toll has been devastating, as we get to over one million infections and 65,000 dead in the US, thankfully, most people have not succumbed to sickness (or death) due to COVID-19, whether infected or not. That’s because most of us have an immune system that protects us from the worst that COVID-19 can deliver.
But what if you or someone you care about does not have a robust immune system, like people in their sixties and beyond?
A particularly problematic feature of this virus is how much more dangerous it is for older adults than for other age groups. Data about COVID-19 infections, hospitalizations, and deaths clearly indicate a much greater risk for older adults.
A recent article from STAT News highlights some of the data from China:
- More than 18% of people in their 80s require hospitalization from COVID-19; whereas
- Only 3.4% of people in their 30s need to be hospitalized.
This begs the question:
How do known changes that occur to the immune system as we age add to the severity of COVID-19 in older adults, and what can be done about it?
That’s the focus on this post.
Read on (and watch the video)…
Contents
How Your Immune System Works
Our immune system is a collection of biological processes that protect against disease by identifying and killing pathogens and tumor cells. It detects a bewildering variety of potentially harmful assaults, from viruses to parasitic worms, and must distinguish them from our own healthy cells and tissues in order to differentiate between friend and foe.
This act of detection is complicated, because pathogens can evolve rapidly and produce adaptations that avoid the immune system, thereby enabling the pathogens to successfully infect us, their hosts.
To meet this challenge, our immune system has evolved to recognize and neutralize pathogens that their cohort of proteins, cells, organs and tissues, which together interact in an elaborate and dynamic network.
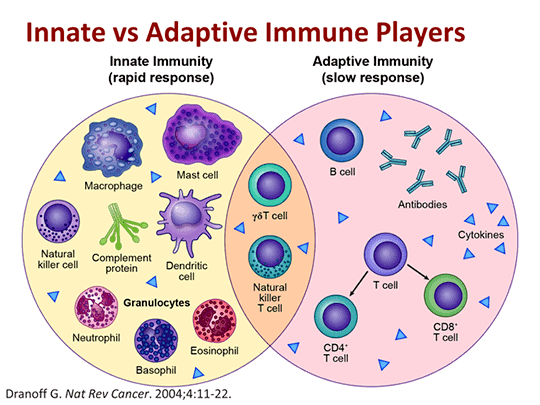
The Innate and Adaptive Response
As part of this more complex immune response, the human immune system adapts over time to recognize specific pathogens more efficiently. This adaptation process is referred to as “adaptive immunity” or “acquired immunity” and creates immunological memory.
Immunological memory created from a primary response to a specific pathogen, provides an enhanced response to secondary encounters with that same, specific pathogen. This process of acquired immunity is the basis of vaccination.
Thus, our immune system operates on two levels:
- the innate response; and
- the adaptive response.
The innate immune system has a generic response to anything it recognizes as “foreign”. It tells the adaptive immune system when it’s time to help mount a defense. One of the primary generic responses of the innate immune system is inflammation, a cellular response that alerts the rest of the system that there’s a problem.
The adaptive immune system responds more slowly than the innate system, but its response is tailored to the specific pathogens the immune system seeks to quell. As the name suggests, in this case, the immune system “adapts” over time to recognize specific pathogens more efficiently. The purpose of this adaptation process is to create immunological memory.
Vaccines typically work by activating the adaptive immune system and “training” the body to recognize a specific virus, a tiny bit of it which is in the vaccine, so the body can adapt to it and easily fight it off if it ever encounters the real thing in higher quantities. The adaptive immune system is also the reason we become immune to some viruses after having them once; the next time our system encounters the virus it is able to quickly tailor an attack to fend off the assault.
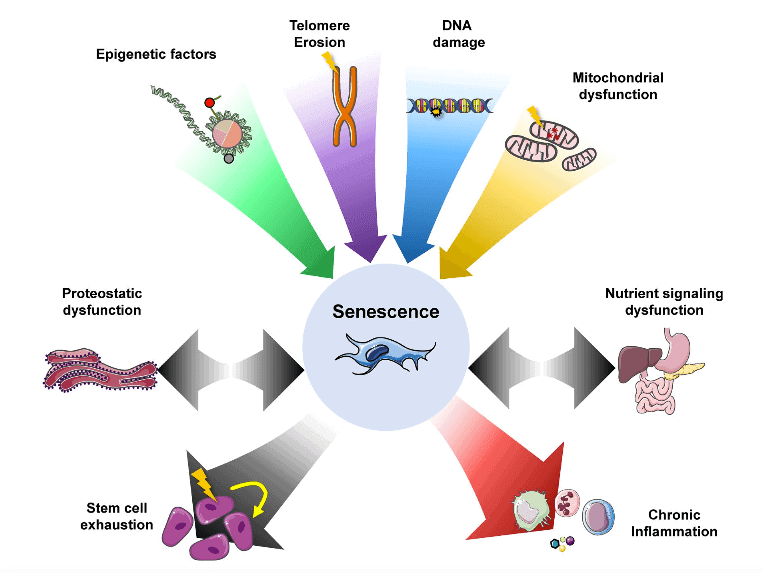
Both aging and underlying disease lead to a gradual deterioration of the immune system, known as “immunosenescence.”
The Effects of Age On the Immune Response
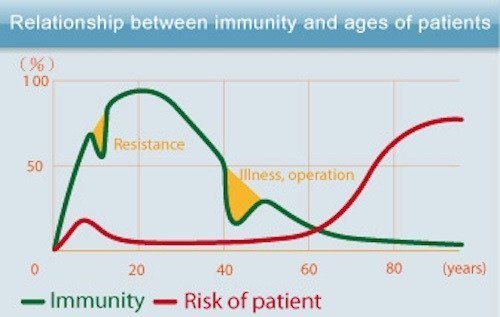
As we age, our immune defense response capability against pathogens becomes reduced, which in turn contributes to more infections and more cancer. As life expectancy in developed countries has increased, so has the incidence of age-related conditions.
Compared with younger people, the elderly are more likely to contract and die from infectious diseases. Respiratory infections, influenza, the COVID-19 virus, and especially pneumonia, are a leading cause of death in people over 65 worldwide.
The reason for this is uncertain, but some scientists believe that we succumb to disease more frequently as we age because the thymus shrinks and produces fewer T-cells to fight infection. Also known as T-lymphocytes ( the “T” stands for “thymus), T-cells are a type of white blood cell that’s at the core of adaptive immunity. T-cells function to actively destroy infected cells, as well as to signal other immune cells to participate in the immune response.
Whether this decrease in thymus size and function explains the drop in T cells, or whether other changes play a role is not fully understood. For instance, it’s possible that as we get older, the bone marrow becomes less efficient at producing the stem cells that give rise to the cells of the immune system.
There appears to be a connection between nutrition and immunity in the elderly. A form of malnutrition that is surprisingly common even in affluent countries is known as “micronutrient malnutrition.” Micronutrient malnutrition, in which a person is deficient in some essential vitamins and trace minerals that are obtained from or supplemented by diet, can happen in the elderly.
Older people tend to eat less and often have less variety in their diets. One important question is whether dietary supplements may help older people, and everyone else, maintain a healthier immune system. I present some evidence that certain supplements, such as vitamins, medicinal mushrooms and herbs, can improve immunity in my article: How To Boost Your Immune System To Fight Viruses Like Coronavirus.
The Aging Impact On Innate and Adaptive Immunity
Like many of our biological systems, the innate and adaptive immune systems change with age, and not for the better.
The aging human immune system is characterized by two key changes:
- An increase in innate immune responses or chronic inflammation — commonly referred to as “inflammaging”, given the close association between aging and systemic (chronic) inflammation.
- A decrease in adaptive responses — referred to as “immunosenescence”, which is a gradual deterioration of the immune system.
An increase in innate immunity, chronic inflammation or inflammaging is characteristic of aging and of the chronic diseases of aging. This leads to damage to inflamed tissues and thus decreases their resilience to infection. It appears that a key factor determining a poor prognosis during COVID-19 is a hyperactive innate immune system, thereby possibly explaining the increased complications in older adults.
A decrease in adaptive responses means that those immune cells that create and hold memories of pathogens die off without dividing. This limits the body’s ability to respond to new infections like this novel coronavirus. This also limits our ability to respond to vaccines as we age.
Eric Verdun, MD is the President and CEO of The Buck Institute for Research on Aging, and also runs a lab dedicated to finding ways to help the immune system maintain its function as it ages. By studying how immune aging is regulated by nutrition, Dr. Verdun’s lab has demonstrated how changes in the relative abundance of key cellular metabolites such as NAD+, acetylcoenzyme A, and the ketone body beta-hydroxybutyrate fluctuate under different nutritional conditions (obesity, calorie restriction, fasting, time-restricted feeding, ketogenic diet), and how this influences immune responses. I’ll return to NAD+ again below.
Your Aging Immune System and COVID-19
There are years of research ahead to fully understand how COVID-19 interacts with an aging immune system, but the evidence already points to a role for both immunosenescence and inflammaging in the increased risk older adults are experiencing. It’s also becoming clear that not only one’s age, but also the presence of pre-existing chronic conditions play a role in the severity of COVID-19.
COVID-19 May Target Senescent Cells
Given that COVID-19 shows a considerably higher mortality rate in patients with advanced chronological age, is there a functional association between COVID-19 infection and the process of chronological aging?
That’s the question proffered in a review of research on the topic published by the journal Aging. And the answer is: It sure looks like it.
Two host receptors have been proposed for COVID-19; meaning, two places on a cell’s surface where COVID-19 can penetrate, replicate and spread.. One is CD26 and the other is ACE-2 (angiotensin-converting enzyme 2).
You may have heard about ACE-2 in the context of ACE-2 inhibitors, which are various pharmaceutical drugs used primarily for the treatment of hypertension (elevated blood pressure) and congestive heart failure. ACE inhibitors prevent an enzyme in your body from producing angiotensin II (ACE-2), a substance in your body that narrows your blood vessels and releases hormones that can raise your blood pressure. This narrowing can cause high blood pressure and force your heart to work harder.
Undoubtedly, you’ve heard less about CD26, perhaps because it’s bewilderingly hard to describe. Very basically, CD26 is a type of enzyme important in human T-cell function that serves as the binding protein to adenosine deaminase (another enzyme that functions in humans in the development and maintenance of the immune system). Let’s just leave it at that.
CD26 joins the conversation on cellular senescence, given that both CD26 and the angiotensin system (ACE-2) show associations with senescence — cells that can no longer divide, but are not dead either. Neither dead nor alive is the characteristic that gives them their nickname, “zombie cells”. Unfortunately, the older we get, the more of these cells we get.
The problem with zombie cells is that their secretions can damage DNA and promote chronic inflammation, among other nefarious effects. But there may be a solution — senolytics.
Senolytics and NAD – two bedfellows for an aging immune system?
Senolytics and NAD aren’t often expressed in the same sentence, unless the sentence is something like:
What are two near-proven ways to extend human healthspan?
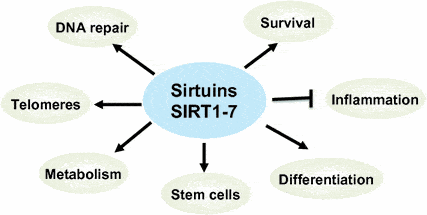
But in this discussion about improving immunity in the older population facing greater COVID-19 exposure as we’re getting closer to re-entering society, there are good reasons to examine why and how to use senolytics to eliminate some zombie cells, and to increase NAD levels, given its role in activating sirtuins, our anti-aging genes.
Senolytics Reduce Senescence
UPDATE (5/7/22): As I explain here, a new study casts shade over the effectiveness of fisetin as a senolytic.
Senolytics is a class of drugs and supplements that selectively eliminate senescent cells. As I wrote in Three Senolytic Drugs Available Now, you can get three off-the-shelf supplements that have been shown to reduce cellular senescence (zombie cells):
- Quercetin, a plant polyphenol from the flavonoid group;
- Piperlongumine, a natural product derived from the fruit of the Asian Long pepper; and
- Fisetin, a flavonoid and antioxidant.
The Aging study mentioned above asserts that the fight against COVID-19 disease should involve testing the hypothesis that senolytics and other anti-aging drugs may have a prominent role in preventing the transmission of the virus, as well as aid in its treatment.
In the video below, Michael Lisanti, Professor at the University of Salford and Specialty Chief Editor of Frontiers in Oncology – Cancer Metabolism, shows us how SARS-CoV-2 may target senescent cells, which become more common in the body as it ages, and how this mode of infection could potentially inform molecular mechanisms and targets for COVID-19 treatment.
NAD Activates Sirtuins, Our Anti-aging Genes
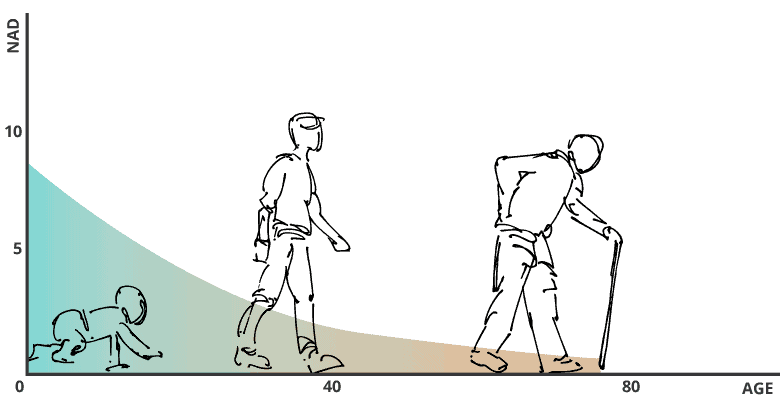
NAD is the acronym for Nicotinamide adenine dinucleotide, a vital coenzyme found in all living cells. Its key role is to convert nutrients into cellular energy (ATP), which is essential for cell health and function. It also supports DNA health and repair. But what too often gets too little attention about the value of keeping NAD levels in the body high, is that it keeps our sirtuins activated.
Dr. Verden’s lab at the Buck Institute is interested in how NAD can stimulate an aging immune system. The lab is focused on developing biomarkers that can spot potential trouble for the immune system, and creating therapeutics that can prevent or reverse damage are a really exciting prospect for improving health and resilience in older adults.
Improving NAD levels is an important focus for the lab, because our levels this coenzyme drops quickly as we age:
That NAD is essential, declines with age and can be boosted with certain precursors has created high demand for such precursors, like NR (nicotinamide riboside) and NMN (nicotinamide mononucleotide). No studies I’m aware of show a direct link between boosting NAD and improving immunity to COVID-19, but it’s something to keep on your radar.
If NAD can be elevated with its precursors — and in animal models and one human trial, it is — our set of anti-aging genes, called sirtuins, can remain activated as we age and lessen many unwanted effects of aging.
For more about NAD, NR, NMN and sirtuins, read:
- The Best 2 NAD Precursors To Extend Your Healthspan
- Dr. David Sinclair and Rhonda Patrick: How NAD and Trans-Resveratrol Activate Your Longevity Genes
- Can NAD+ Precursors NR and NMN Make You Young Again?
Your Takeaway
Remember these five things:
- Your immune system is exquisitely designed to protect you from infections and disease, but it’s never met COVID-19 before, so both the innate and adaptive mechanisms of immunity are challenged when this infection occurs.
- Despite the novelty of COVID-19, for most of us, our immune system fights off infection and/or substantially reduces the severity of the symptoms.
- But that’s not true for older people, because like many biological systems that gradually become impaired as we age, so does our immune system; in fact, COVID-19 appears to be especially problematic for older folk, due to how it infects them.
- Various nutrients and supplements thought to improve immunity for influenza, and therefore COVID-19 (see them here).
- The precursors NR and NMN that increase NAD levels (and thereby activate sirtuins) and senoyltics (drugs and supplements) that reduce zombie cells may also be effective therapies to improve the immune system of the elderly.
Last Updated on May 7, 2022 by Joe Garma