Understanding Senescence and Senolytics: A Comprehensive Overview
Understanding senescence and senolytcs is the first step to doing something about slowing down the aging process that inevitably increases the risk of serious health issues like heart disease, cognitive decline, and cancer. The goal here is to address one root cause of age-related health problems by targeting the accumulation of senescent cells. By using senolytics, researchers aim to promote healthier aging and possibly extend the period of life characterized by good health.
To understand senescence and senolytics is to get a good understanding of how we age and what to do about slowing down the pace of aging.
Surely, it’s in your vested interest to do so.
As our world’s population continues to age, the challenges posed by age-related diseases are becoming increasingly apparent. Conditions like cardiovascular disease, neurodegenerative disorders, and cancer are on the rise, correlating with the advancing years.
Within this complex landscape of aging and disease, cellular senescence has emerged as a key player. Cellular senescence refers to a state where cells undergo a permanent halt in their cell cycle due to various stressors, accompanied by the secretion of pro-inflammatory molecules.
This phenomenon, while initially thought to be a protective response, has been implicated in various chronic diseases, such as type 2 diabetes, osteoarthritis, and even the severity of SARS-CoV-2 infection.
Here, I delve into a synthesis of four studies (#1, #2, #3, and #4) that collectively shed light on cellular senescence, its role in aging, and the emerging therapeutic strategies known as senolytics:
- Study #1: Emerging senolytic agents derived from natural products
- Study #2: Senolytic drugs: from discovery to translation
- Study #3: Discovery of new senolytics using machine learning
- Study #4: First evidence that senolytics are effective at decreasing senescent cells in humans
The objective of this review about senescence and senolytics is to give you:
- An appreciation of how relevant and important they are to understanding how we age; and
- By covering some of the scientifically-validated senolyetics, to provide you with over-the-counter available compounds that might help reduce your senescent burden.
Let’s dig in…
Understanding Cellular Senescence: The Underlying Mechanisms
Cellular senescence is a hallmark of aging, it is a permanent state of cell cycle arrest induced by cellular stresses, which refers to a condition where a cell stops dividing and progressing through its normal cycle due to various types of stress factors.
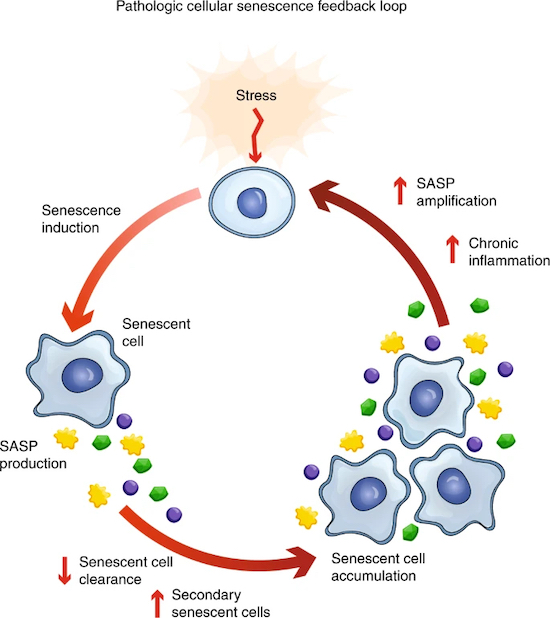
In response to stressors such as DNA damage, oxidative stress, or other cellular insults, a cell can enter a state known as cellular senescence. In this state, the cell no longer goes through the stages of the cell cycle – including phases like DNA replication and cell division – and remains in a sort of “standby” mode. This arrest in the cell cycle is “permanent” because the cell does not revert back to its normal cycle even after the stress is resolved. Instead, the cell typically undergoes specific changes in gene expression and secretes inflammatory molecules, contributing to tissue dysfunction and the development of age-related diseases.
Cellular senescence is characterized by a permanent cell cycle arrest and the release of a proinflammatory secretome known as the senescence-associated secretory phenotype (SASP). These are a group of molecules, including proteins and inflammatory factors, that are secreted by senescent cells. Such substances can have harmful effects on surrounding cells and tissues, promoting inflammation and contributing to age-related diseases.
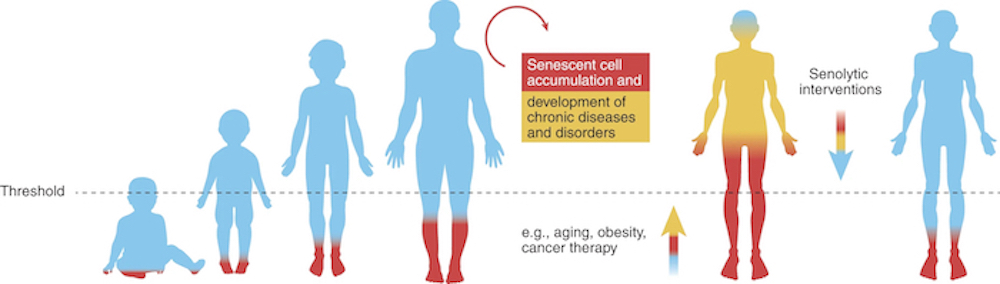
Initially recognized as a tumor suppressor mechanism, senescence has been implicated in the aging process and a host of disease pathways that are common to aging. As we get older, cellular senescence becomes more prevalent across various tissues, contributing to the development of numerous chronic conditions.
Damaged cells exit the normal cell cycle and become senescent, releasing SASP factors that can disrupt tissue integrity and function. This has significant implications, even when senescent cells are present in relatively low abundance – as little as 10-15% in aged primates, such as ourselves – they can still have profound effects on tissue dysfunction [1].
As you’ll soon see, this accumulation of senescent cells sets the stage for the search for novel therapeutic strategies that can target and eliminate these cells, potentially alleviating age-related diseases.
Senescence and Senolytics: Targeting Senescent Cells for Better Aging
Enter senolytics, the innovative class of therapeutics (pharmacological drugs and/or nutraceuticals) designed to selectively clear senescent cells from the body.
In Study #1: Emerging senolytic agents derived from natural products, the potential of natural compounds as senolytic agents is explored. This area of research introduces a fascinating dimension to senolytics – the use of naturally occurring substances as therapeutic interventions. Compounds like quercetin, fisetin, piperlongumine, and curcumin analogs have demonstrated senolytic properties in animal models. These compounds have the potential to extend beyond the laboratory and enter clinical applications.
Click here for the Study #1 abstract
Cellular senescence is a hallmark of aging, it is a permanent state of cell cycle arrest induced by cellular stresses. During the aging process, senescent cells (SCs) increasingly accumulate in tissues, causing a loss of tissue-repair capacity because of cell cycle arrest in progenitor cells and produce proinflammatory and matrix-degrading molecules which are known as the senescence-associated secretory phenotype (SASP), and thereby contribute to the development of various age-related diseases. Genetic evidence has demonstrated that clearance of SCs can delay aging and extend healthspan. Senolytics, small molecules that can selectively kill SCs, have been developed to treat various age-related diseases. In recent years, emerging natural compounds have been discovered to be effective senolytic agents, such as quercetin, fisetin, piperlongumine and the curcumin analog. Some of the compounds have been validated in animal models and have great potential to be pushed to clinical applications. In this review, we will discuss cellular senescence and its potential as a target for treating age-related diseases, and summarize the known natural compounds as senolytic agents and their applications.
As Study #2: Senolytic drugs: from discovery to translation reports, senolytics hold promise as a “hit-and-run” intervention, temporarily disabling pro-survival pathways in senescent cells and triggering their apoptosis, or programmed cell death. This unique approach aims to reduce the burden of senescent cells in various tissues, potentially ameliorating a broad spectrum of age-related conditions.
Click here for the Study #2 abstract
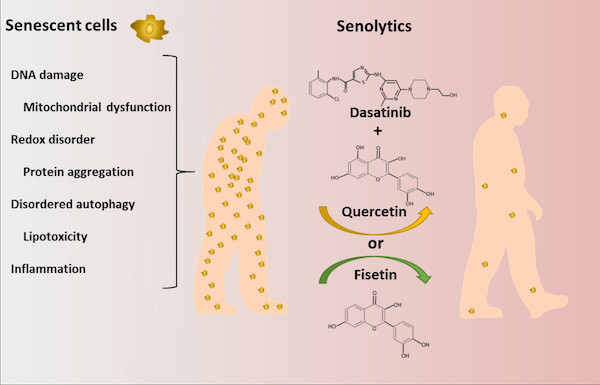
Senolytics are a class of drugs that selectively clear senescent cells (SC). The first senolytic drugs Dasatinib, Quercetin, Fisetin and Navitoclax were discovered using a hypothesis‐driven approach. SC accumulate with ageing and at causal sites of multiple chronic disorders, including diseases accounting for the bulk of morbidity, mortality and health expenditures. The most deleterious SC are resistant to apoptosis and have up‐regulation of anti‐apoptotic pathways which defend SC against their own inflammatory senescence‐associated secretory phenotype (SASP), allowing them to survive, despite killing neighbouring cells. Senolytics transiently disable these SCAPs, causing apoptosis of those SC with a tissue‐destructive SASP. Because SC take weeks to reaccumulate, senolytics can be administered intermittently – a ‘hit‐and‐run’ approach. In preclinical models, senolytics delay, prevent or alleviate frailty, cancers and cardiovascular, neuropsychiatric, liver, kidney, musculoskeletal, lung, eye, haematological, metabolic and skin disorders as well as complications of organ transplantation, radiation and cancer treatment. As anticipated for agents targeting the fundamental ageing mechanisms that are ‘root cause’ contributors to multiple disorders, potential uses of senolytics are protean, potentially alleviating over 40 conditions in preclinical studies, opening a new route for treating age‐related dysfunction and diseases. Early pilot trials of senolytics suggest they decrease senescent cells, reduce inflammation and alleviate frailty in humans. Clinical trials for diabetes, idiopathic pulmonary fibrosis, Alzheimer’s disease, COVID‐19, osteoarthritis, osteoporosis, eye diseases and bone marrow transplant and childhood cancer survivors are underway or beginning. Until such studies are done, it is too early for senolytics to be used outside of clinical trials.
The first generation of senolytics, such as dasatinib, quercetin, fisetin, and navitoclax, was identified through a hypothesis-driven approach.
Dasatinib
Dasatinib is a pharmacological drug classified as a tyrosine kinase inhibitor. Originally developed as a targeted therapy for certain types of leukemia, dasatinib has shown potential as a senolytic agent. In the context of senolytics, it is used to selectively induce the death of senescent cells, contributing to the reduction of their burden in tissues. This approach aims to alleviate age-related conditions by eliminating dysfunctional cells.
Quercetin
Quercetin is a natural compound classified as a flavonoid, found in various fruits, vegetables, and plants. It has gained attention for its potential health benefits, including anti-inflammatory and antioxidant properties. In the field of senolytics, quercetin has shown promise in targeting senescent cells and promoting their clearance. It is considered a senolytic agent that can help remove these cells, potentially improving tissue function and mitigating age-related disorders.
Fisetin
Fisetin is another natural flavonoid found in certain fruits and vegetables, particularly strawberries. Like quercetin, fisetin possesses antioxidant and anti-inflammatory properties. In the context of senolytics, fisetin has demonstrated senolytic effects in animal studies. It is being explored as a potential agent for targeting senescent cells and enhancing tissue health, thereby contributing to healthy aging.
Navitoclax
Navitoclax is a synthetic pharmacological drug categorized as a Bcl-2 family inhibitor. Originally developed as an anti-cancer agent, navitoclax has shown potential as a senolytic compound. It functions by disrupting pro-survival pathways in senescent cells, leading to their apoptosis or programmed cell death. This selective elimination of senescent cells holds promise for improving age-related health issues by reducing their detrimental effects on tissues.
These therapeutics collectively represent a diverse array of approaches to tackling cellular senescence, with the aim of promoting healthier aging and potentially extending the period of good health in individuals. They have shown efficacy in eliminating senescent cells in animal models, improving physical function, and extending health span.
The senolytics field is expanding (see below), with ongoing clinical trials for various conditions, including diabetes, idiopathic pulmonary fibrosis, Alzheimer’s disease, and osteoarthritis.
Exploring Novel Senolytics: Advances in Drug Discovery

Credit: https://today.uconn.edu/2019/04/drug-discovery-partnership-ai-biotech-company-reaps-promising-early-results/
Study #3: Discovery of new senolytics using machine learning introduces an exciting development in the field of senolytics – the application of machine learning for the discovery of novel senolytic compounds. Researchers have harnessed the power of existing drug screening data to train machine learning algorithms in classifying compounds based on their senolytic activity. This approach offers a cost-effective and efficient way to identify potential senolytics.
Click here for the Study #3 abstract
Cellular senescence is a stress response characterised by a permanent cell cycle arrest and a proinflammatory secretome. In addition to its tumour suppressor role, senescence is involved in ageing and promotes many disease processes such as cancer, type 2 diabetes, osteoarthritis, and SARS-CoV-2 infection. There is a growing interest in therapies based on targeted elimination of senescent cells, yet so far only a few such senolytics are known, partly due to the poor grasp of the molecular mechanisms that control the senescence survival programme. Here we report a highly effective machine learning pipeline for the discovery of senolytic compounds. Using solely published data, we trained machine learning algorithms to classify compounds according to their senolytic action. Models were trained on as few as 58 known senolytics against a background of FDA-approved compounds or in late-stage clinical development (2,523 in total). We computationally screened various chemical libraries and singled out top candidates for validation in human lung fibroblasts (IMR90) and lung adenocarcinoma (A549) cell lines. This led to the discovery of three novel senolytics: ginkgetin, oleandrin and periplocin, with potency comparable to current senolytics and a several hundred-fold reduction in experimental screening costs. Our work demonstrates that machine learning can take maximum advantage of existing drug screening data, paving the way for new open science approaches to drug discovery for senescence-associated diseases.
The results are three novel senolytic compounds: ginkgetin, oleandrin, and periplocin.
Ginkgetin
Ginkgetin is a natural compound derived from the leaves of the Ginkgo biloba tree, which is known for its potential cognitive and antioxidant benefits. In the context of senolytics, ginkgetin has shown promise as a senolytic agent. It is being investigated for its ability to selectively target and eliminate senescent cells, potentially contributing to the mitigation of age-related diseases and improvements in tissue function.
Oleandrin
Oleandrin is a natural compound extracted from the oleander plant. Historically, it has been used in traditional medicine, but it’s also known for its toxic properties. In recent times, oleandrin has garnered attention as a potential therapeutic compound, including as a senolytic agent. It has been studied for its ability to induce apoptosis, or programmed cell death, in senescent cells, thereby reducing their burden and potential negative impact on health.
Periplocin
Periplocin is a compound derived from plants of the genus Periploca. It has been investigated for its potential health benefits, including anti-inflammatory and anti-cancer properties. In the context of senolytics, periplocin has shown promise in targeting senescent cells. It has been studied for its ability to induce apoptosis in these cells, potentially contributing to healthier aging and reduced susceptibility to age-related diseases.
These compounds have demonstrated comparable potency to existing senolytics and have the potential to significantly reduce the cost of experimental screening. This innovative use of machine learning represents a step forward in the quest for effective interventions to combat senescence-associated diseases.
The 7 Senolytics Reviewed
I described above the seven senolytics covered in the four studies summarized in this post. Now I’ll categorize them by pharmaceutical drugs, for which you would need a medical doctor’s prescription to obtain and use, and by compounds that can be purchased without a prescription.
I will also indicate which one I use; however, do understand that I have no idea if any of them are reducing my senescent cell burden. Also, know that I cycle in and out of taking them, given that there’s some indication that you do not want to try to eliminate all senescent cells, as they do have a beneficial role to play [2].
I implore you to talk to your doctor before taking any of these nutraceuticals and to know about the side effects, if any, and also to suggest doseage.
Pharmaceutical drugs
1. Dasatinib is a pharmacological drug classified as a tyrosine kinase inhibitor.
2. Navitoclax is a synthetic pharmacological drug categorized as a Bcl-2 family inhibitor.
Nutraceuticals
3. Quercetin is a natural compound classified as a flavonoid, found in various fruits, vegetables, and plants. (I use this intermittingly.) ConsumerLab top pick available on Amazon.
4. Fisetin is another natural flavonoid found in certain fruits and vegetables, particularly strawberries. (I use this intermittingly.) Lipo Fisetin by Renue by Science was shown to be up to 47 times more bioavailable than free fisetin, according to ConsumerLab.
5. Ginkgetin is a natural compound derived from the leaves of the Ginkgo biloba tree, which is known for its potential cognitive and antioxidant benefits. Life Extension Ginkgo Biloba is ConsumerLab’s top pick.
6. Oleandrin is a natural compound extracted from the oleander plant, but it’s also known for its toxic properties. I found no third-party review of this nutracuetical.
7. Periplocin is a compound derived from plants of the genus Periploca. It has been investigated for its potential health benefits, including anti-inflammatory and anti-cancer properties. I found no third-party review of this nutracuetical.
Note: I also take piperlonguminine, which was not reviewed in the four studies examined here., but I cover piperlonguminine, as well as some more about quercetin and fisetin in my post: Can New Senolytics Drugs Delay Aging? Part 2: Three Senolytic Drugs Available Now.
The Road Ahead: Challenges and Possibilities
While the potential of senolytics is promising,
Study #4: First evidence thatsenolytics are effective at decreasing senescent cells in humans highlights the need for caution.
Despite encouraging results from preclinical studies, questions remain about the long-term effects of senolytic drugs. Researchers are investigating various aspects, such as potential side effects, responsiveness of different cell types to senolytics, and the impact of clearing post-mitotic cells in organs with limited regenerative capacity. These concerns emphasize the importance of rigorous clinical trials to assess both safety and efficacy.
Click here for the Study #4 abstract
The increasing life expectancy of the world population has become an economic and global public health problem. Increased life expectancy tracks with a higher incidence of multiple chronic conditions, despite unprecedented advances in prevention, diagnostics, and treatment. Ageing is the greatest risk factor for many life-threatening disorders, including cardiovascular disease, neurodegeneration and cancer. Ageing in all tissues is associated with increased cellular senescence, a stress-response process whereby damaged cells exit the cell cycle permanently and produce a pro-inflammatory senescenceassociated secretory phenotype (SASP). Moreover, senescent cells accumulate in multiple chronic diseases across the age range, like Obesity and Chronic Kidney Disease (CKD). Long-term persistence of senescent cells and their SASP disrupt tissue structure and function having deleterious paracrine and systemic effects causing fibrosis, inflammation, and a possible carcinogenic response. Remarkably, even a relatively low abundance of senescent cells (10 15% in aged primates) [1] is sufficient to cause tissue dysfunction [2]. Prof. James Kirkland and his team at Mayo Clinic have pioneered a new class of agents which eliminate senescent cells named ‘senolytics’ from the words “senescence” and “lytic” destroying. Through exploiting senescent cells’ dependence on specific pro-survival pathways, senolytics transiently disable the pro-survival networks that defend senescent cells against their own apoptotic environment without affecting proliferating or quiescent, differentiated cells [3,4]. Senolytics thus far tested include dasatinib (D, a FDAapproved tyrosine kinase inhibitor), quercetin (Q, a flavonoid present in many fruits and vegetables), navitoclax, A1331852 and A1155463 (Bcl-2 pro-survival family inhibitors) and fistein (F, a flavonoid) [3]. Pre-clinical studies conducted in mice have shown senolytics eliminate senescent cells resulting in delaying, preventing or alleviating multiple ageand senescence-related conditions, including The increasing life expectancy of the world population has become an economic and global public health problem. Increased life expectancy tracks with a higher incidence of multiple chronic conditions, despite unprecedented advances in prevention, diagnostics, and treatment. Ageing is the greatest risk factor for many life-threatening disorders, including cardiovascular disease, neurodegeneration and cancer. Ageing in all tissues is associated with increased cellular senescence, a stress-response process whereby damaged cells exit the cell cycle permanently and produce a pro-inflammatory senescenceassociated secretory phenotype (SASP). Moreover, senescent cells accumulate in multiple chronic diseases across the age range, like Obesity and Chronic Kidney Disease (CKD). Long-term persistence of senescent cells and their SASP disrupt tissue structure and function having deleterious paracrine and systemic effects causing fibrosis, inflammation, and a possible carcinogenic response. Remarkably, even a relatively low abundance of senescent cells (10À15% in aged primates) [1] is sufficient to cause tissue dysfunction [2] . Prof. James Kirkland and his team at Mayo Clinic have pioneered a new class of agents which eliminate senescent cells named ‘senolytics’ -from the words “senescence” and “lytic” À destroying. Through exploiting senescent cells’ dependence on specific pro-survival pathways, senolytics transiently disable the pro-survival networks that defend senescent cells against their own apoptotic environment without affecting proliferating or quiescent, differentiated cells [3, 4] . Senolytics thus far tested include dasatinib (D, a FDAapproved tyrosine kinase inhibitor), quercetin (Q, a flavonoid present in many fruits and vegetables), navitoclax, A1331852 and A1155463 (Bcl-2 pro-survival family inhibitors) and fistein (F, a flavonoid) [3] . Pre-clinical studies conducted in mice have shown senolytics eliminate senescent cells resulting in delaying, preventing or alleviating multiple age-and senescence-related conditions, including frailty, cataracts, age-related osteoporosis, age-related muscle loss, radiation-induced damage, cardiac dysfunction, vascular dysfunction and calcification, pulmonary fibrosis, hepatic steatosis, metabolic syndrome, diabetes and dementia [3À5]. Overall, in mice, administration of senolytic agents and elimination of senescent cells have shown to improve physical function and extend health span and lifespan [2, 6] . The results of the first-in-human, single-arm, open-label clinical trial of senolytics were published early this year in this Journal [7] . Subjects with idiopathic pulmonary fibrosis, a cellular senescencedriven fatal disease, showed significantly improved walking endurance, gait speed, chair rise test performance, and scores in the Short Physical Performance Battery 5 days after 9 doses of D + Q over 3 weeks [7] . Published recently in EBioMedicine, Kirkland and colleagues, demonstrate for the first time that a short (3 day) course of senolytics D + Q (D: 100 mg/day; Q: 500 mg twice daily) decrease senescent cells in humans with drug-controlled diabetes mellitus and CKD (age range 55À79 years old) [8] . In this ongoing clinical trial, whereby the effects of D + Q senolytic therapy on alleviating tissue dysfunction and disease progression in diabetes and chronic kidney disease (CKD) in humans are being tested, blood samples, adipose tissue and skin biopsies were taken before and 11 days after the short course of D + Q. Markers of senescent cells, p16 INK4A , p21 CIP1 and SAbgal, were reduced by 35%, 17% and 62% in abdominal subcutaneous adipose tissue, respectively. Senescent cells attract, activate and anchor macrophages and a 28% decrease was found in CD68+ macrophages in adipose tissue following D + Q. The replicative potential of primary adipocyte progenitor cells, isolated from the adipose tissue, increased over time following D + Q. This increased replicative potential following senescent cell clearance using D + Q is like that seen with tissue-resident human cardiac progenitor cells in vitro [9] . In addition to adipose tissue, D + Q reduced p16 INK4A -and p21 CIP1 -positive cells by 38% and 30% in the epidermal layer of the skin, respectively. Finally, key circulating SASP factors (IL-1a, À2, À6, and À9 and Matrix Metalloproteinases (MMP) À2, À9, and À12) were significantly lower 11 days after than before the 3 days of D + Q. Senescent cells take weeks to over a month to form and acquire a SASP. The findings suggest that senolytics given intermittently in a ‘hit-and-run’ fashion, despite the elimination half-lives of D and Q being <11 h, is effective at reducing senescent cell burden and could lessen side effects, which can occur when D is administered continuously. Further randomised, controlled clinical trials using different senolytics are underway or planned. For example, the Translational Geroscience Network, headed by the Kirkland team at the Mayo Clinic, will conduct senolytic clinical trials targeting fundamental aging mechanisms to extend healthspan and delay, prevent, or treat age-and cellular senescence-related conditions. The findings from these trials will not only determine the safety and efficacy of senolytics, but they could be transformative in the care and treatment of older adults and patients with multiple chronic diseases. However, despite these exciting preliminary translational developments in the senolytics field we should still err on the side of caution. There are many unanswered questions. As well as the unknown short-and long-term side effects of individual senolytics drugs, we need to understand which and how individual senescent cell types contribute to tissue dysfunction, are all cell types equally responsive to senolytics, could senolytics have detrimental effects in otherwise healthy cells, and could clearance of post-mitotic cells in organs with limited regenerative capacity like the heart and brain, lead to detrimental effects by interfering with tissue integrity.
Your Takeaway on Senescence and Senolytics
The landscape of aging and age-related diseases is complex, involving a multitude of cellular processes. Cellular senescence has emerged as a critical factor in this equation, contributing to chronic conditions and tissue dysfunction.
Senolytics represent a novel and innovative approach to addressing these issues. By selectively targeting and eliminating senescent cells, senolytics have the potential to improve health span, extend the period of healthy aging, and alleviate a range of age-related conditions.
As researchers navigate the challenges and opportunities presented by senolytics, ongoing clinical trials and the exploration of natural compounds open new avenues for future interventions. With a careful balance of research, innovation, and caution, the field of senolytics holds promise for revolutionizing our approach to aging and age-related diseases.
Last Updated on August 13, 2023 by Joe Garma