The Aging Response — How You React to Aging and What To Do About It, Part 2


Image credit: Crvitality.com
This piece on aging response hallmarks follows on the heels of Part 1, The 4 Reasons We Age and What To Do About It. We continue with reviewing Dr. Carlos Lopez-Otin and his team of researchers who authored a seminal paper on aging called, The Hallmarks of Aging.
Whereas in Part 1, the focus was on the Primary reasons we age, here we’ll investigate the three Antagonistic (or Compensatory) and two Integrative reactions to the Primary category. Importantly, we’ll also look at what interventions can be done to slow down the degradation that is aging with the aim of extending our healthspan, if not our lifespan.
This is a long and dense look at the science of aging. If you find yourself faltering, scroll down to the bottom and read the bottom line in “Your Takeaway”.
Here’s what we’re going to cover:
- How the Hallmarks of Aging interrelate.
- Deregulated Nutrient Sensing — how metabolism becomes imbalanced.
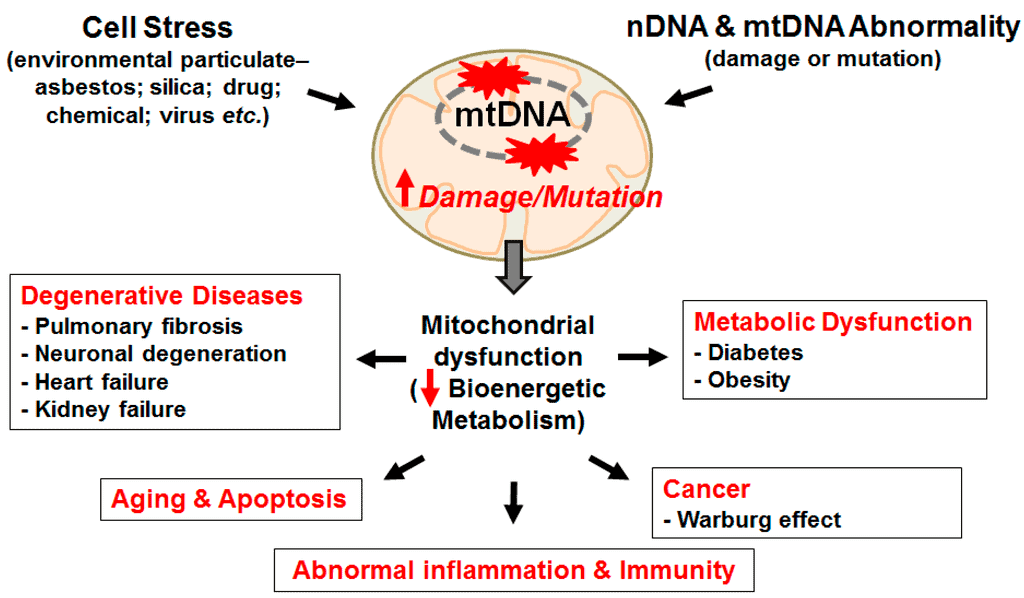
- Mitochondrial Dysfunction — why the body’s energy factory falters.
- Cellular Senesence — what zombie cells do to us.
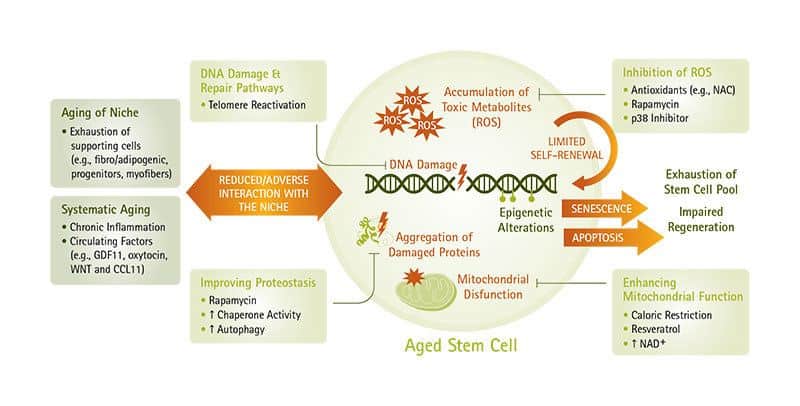
- Stem Cell Exhaustion — no more tissue and cellular renewal.
- Altered Cellular Communication — you just don’t understand me anymore.
In each section, I’ll explain the science a bit and what interventions are available for you to try.
Let’s dig in…
Summary of the Hallmarks of Aging — How they interrelate
As was done in Part 1, it’s helpful to set the stage for what’s to come by quickly reviewing the nine characteristics (hallmarks) of aging as discerned by Dr. Carlos Lopez-Otin and his research team in their classical work, The Hallmarks of Aging.
These hallmarks are shown in this graphic, which show an interconnected, hierarchical relation between them, as discerned by Dr. Carlos Lopez-Otin’s team:
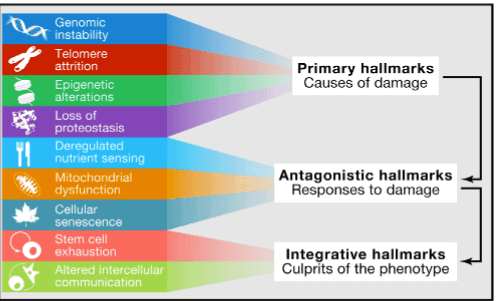
This is Figure 6 from The Hallmarks of Aging study
Primary Hallmarks of Aging
The common characteristic of the Primary hallmarks is that they are all unequivocally negative, and are considered to be the initiating triggers whose damaging consequences progressively accumulate with time.
Part 1 was devoted to the four Primary hallmarks of aging: genomic instability, telomere attrition, epigenetic alterations and loss of proteostasis.
Antagonistic/Compensatory Hallmarks of Aging
In contrast to the Primary, Antagonistic hallmarks have opposite effects depending on their intensity. At low levels, they mediate beneficial effects, but at high levels, they become deleterious.
Here are three examples stemming from their respective hallmark categorization:
- Degregulated nutrient sensing — Optimal nutrient sensing and anabolism are important for survival, but in excess and over time can become pathological.
- Mitochondrial dysfunction — ROS (referring to Reactive Oxygen Species, or “free radicals”) mediate cell signaling and survival, but at chronic high levels can produce cellular damage.
- Cellular senescence — Senescence protects us from cancer, but if excessive can promote aging.
Antagonistic hallmarks can have the capacity to protect us from damage or from nutrient scarcity; however, when exacerbated or chronic, their purpose is subverted and they generate further damage.
Integrative Hallmarks of Aging
The third category comprises the two Integrative hallmarks that directly affect tissue homeostasis and function:
- Stem cell exhaustion, and
- Altered intercellular communication.
These Integrative hallmarks arise when the accumulated damage caused by the Primary and Antagonistic hallmarks overwhelm tissue homeostatic mechanisms (body cell/tissue regulation), and thereby can not give compensating effects.
Now that you have some clarity about how the nine hallmarks of aging relate to each other, the rest of Part 2 will delve into the Compensatory and Integrative hallmarks and suggest various interventions to resist or repair some of the degradation that is aging.
Compensatory Aging Response Hallmarks
Also known as aging response hallmarks, the three compensatory hallmarks are physiological changes which come about as a result of the primary hallmarks. If geroscientists can stop the primary hallmarks of aging, then, in theory, this would eliminate the response hallmarks, making their potential compensatory activities less needed.
What this means: Cells must adapt to nutrient characteristics and quantities. If such adaptions are compromised, a cascade of health issues will occur.
What’s Deregulated Nutrient Sensing? Nutrient sensing is a cell’s ability to recognize and respond to food/fuel such as glucose. It has an essential role in maintaining cellular homeostasis (balance) by regulating downstream metabolic processes. When “deregulated” at least four metabolic pathways are negatively affected.
What you need to know:
- Each type of fuel used by the cell requires an alternate pathway of use and accessory molecules. In order to conserve resources a cell will only produce molecules that it needs at the time. (1)
- As we age, cells become less right at detecting the amount and type of food/fuel available to them. This can affect, for instance, four vital protein groups and their associated pathways; namely: IGF-1, mTOR, sirtuins, and AMPK. These proteins are referred to as “nutrient sensing” because nutrient levels influence their activity. (2)
- Caloric mimicking diets — such as various types of Intermittent Fasting and the Prolon Fasting Mimicking Diet — may improve, or upregulate, nutrient sensing pathways and even reverse diabetes. (3, 4)
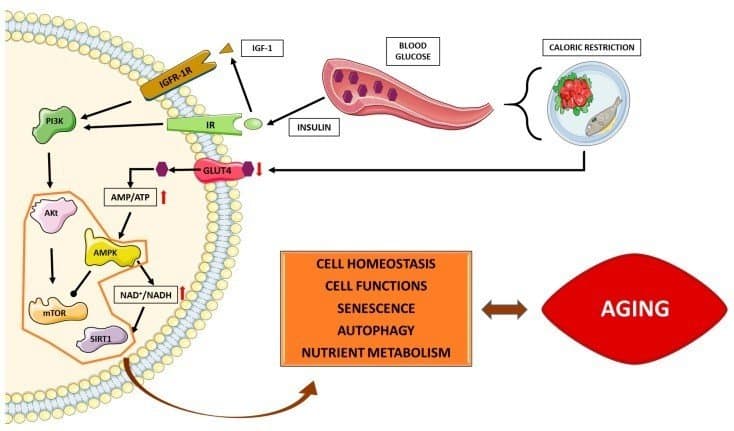
The aging response is a process of degeneration. What we see in our aging bodies is simply the physical manifestation of decades of accumulated damage to our nutrient sensing pathways. Over time, metabolic activities place increasing stress on our cells. Too much food intake or the wrong nutrient composition causes cells to age faster.
Our brain’s hypothalamus also becomes dysfunctional, leading us to eat more than the body needs, potentially resulting in metabolic syndrome, obesity, and type 2 diabetes. To make things worse, obesity and diabetes provoke chronic inflammation, which can further deregulate our nutrient sensing system.
Because metabolic pathways are intertwined with longevity pathways, geroscientists have focused on them in their search for anti-aging treatments. Caloric restriction continues to be a leading anti-aging intervention.
The most studied type of calorie restriction is an indefinite +/- 30% reduction in ingested calories from a baseline amount of calories relative to an organism’s size. Studies with worms, rodents and monkeys have shown an increase of healthspan and lifespan through a sustained reduction of calories. (5, 6, 7)
It’s harder to do longevity studies on long-lived humans than animals/organisms with lifespans of a few weeks (worms) to twenty years (monkeys); however, sustained caloric reduction in humans has been shown to improve various biomarkers associated with aging, such as the inflammatory cytokines profile, adiponectin/leptin ratio, insulin and IGF-1, and thereby is considered a robust life extension protocol. (8)
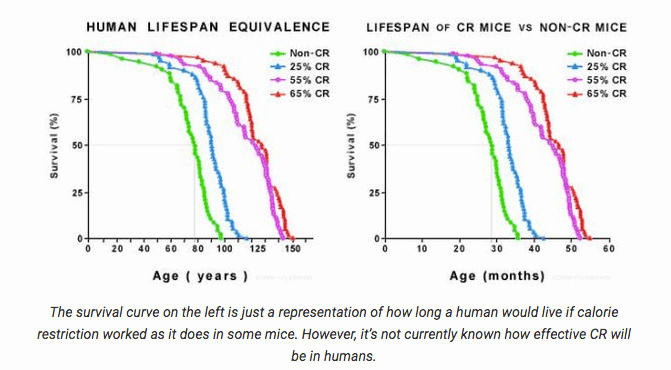
Credit: Crvitality.com
The bad news is that nearly no one is willing to suffer through a lifetime of sustained restriction of caloric intake of 30% or more, no matter what the reward. The good news is that intermittent caloric restriction can “mimic” the health and longevity benefits of sustained calorie deprivation without the difficulty, for it’s easier to restrict calories during intervals than forever.
(For more on the benefits of CR restriction, read this on CR Vitality.)
What You Can Do
As just mentioned, research studies show that caloric restriction and forms of intermittent fasting such as the Fasting Mimicking Diet (FMD) can extend human lifespan, as they do with worms, fruit files, rodents and monkeys.
Dr. Valter Longo is among the world’s top experts on the health and longevity aspects of “fasting diets”. His research has led him to develop the Prolon Fasting Mimicking Diet, which has demonstrated improvements in the aging response of several health and aging-related biomarkers, as well as weight loss. (9, 10, 11)
Mitochondrial dysfunctions during aging are also connected with hormesis, a concept upon which a number of aging research lines have recently converged. (14) According to the hormesis hypothesis (similar conceptually to the therapeutic benefits of inoculations), mild toxic treatments trigger beneficial compensatory responses that surpass the repair of the triggering damage and actually produce an improvement in cellular fitness when compared to the starting pre-damage conditions. Thus, although severe mitochondrial dysfunction is pathogenic (causes disease), mild respiratory deficiencies may increase lifespan, perhaps due to a hormetic response (aka, “what doesn’t kill me makes me stronger”).
There’s compelling evidence that compounds such as metformin and resveratrol are mild mitochondrial poisons that induce a low-energy state characterized by increased AMP levels and activation of AMPK (AMP-activated protein kinase), an enzyme that plays a role in cellular energy homeostasis (balancing). (15)
In mammals, metformin can increase mouse healthspan and lifespan (16), and there’s convincing evidence that various sirtuin activators can protect from metabolic damage and improve mitochondrial respiration. (17, 18)
Since the publication of the Hallmarks of Aging, geroscientists have placed greater attention on the mitochondrial role in the metabolic aging response, with a special focus on mitochondrial sirtuins.
As I wrote in Part 1, Dr. Leonard Guarente and Dr. David Sinclair, among others, have conducted various research studies that show sirtuin-activating NAD (Nicotinamide Adenine Dinucleotide) precursors, NMN (Nicotinamide Mononucleotide) and NR (Nicotinamide Riboside) can enhance mitochondrial function and potentially lead to favorable healthspan and lifespan outcomes. (19, 20)
Endurance training and alternate-day/intermittent fasting may improve healthspan through their capacity to help you avoid mitochondrial degeneration. (x, x) Dr. David C. Rubinsztein and his research team speculate that these beneficial effects are enabled, at least in part, through the induction of autophagy, for which both endurance training and fasting constitute potent triggers. ( 21 )
Read my articles:
Can NAD+ Precursors NR and NMN Make You Young Again?
Get Biologically Younger With A Fast HIIT Feast-Famine Program
Why You Must Exercise for Longevity and How To Do It
How Intermittent Fasting Ignites Cellular Autophagy and A Longer, Healthier Life
3. Cellular senescence — Cells don’t die when they’re supposed to (Antagonistic/Compensatory)
What this means: Cells are stressed and damaged their aging response is to become zombie cells, meaning they are not fully alive nor dead.
What’s Cellular Senescence? It’s when normal cells stop dividing and properly function, but are still alive.
What you need to know:
- In humans, aging promotes a host of degenerative pathologies (diseases) that are characterized by debilitating losses of tissue or cellular function. At the same time, aging also promotes regenerative (hyperplastic) pathologies, such as tumors, the most deadly being cancer.
- Cellular senescence links both of these opposite consequences of aging: This biological phenomenon can both suppress tumor growth (a good thing) and drive degenerative diseases through promoting chronic inflammation (a bad thing and considered among the causes of aging and/or an amplifier of health problems associated with aging). (22)
- You can potentially cut the number of scenescent cells through senolytic and curcumin supplementation.
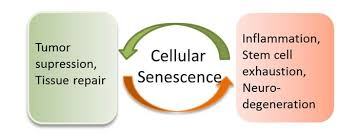
The number of senescent cells increases with aging, leading to the widely held assumption that senescence contributes to the aging response. This view, however, undervalues what is conceivably the primary purpose of senescence — to prevent the propagation of damaged cells and to trigger their demise by the immune system. Therefore, it’s possible that senescence is a beneficial compensatory response that contributes to rid tissues from damaged and potentially oncogenic (cancerous) cells.
But there’s a catch.
Senescent cells’ secretions (secretome) are rich in two types of proteins, the proinflammatory cytokines and matrix metalloproteinases (referred to as the ‘‘senescence-associated secretory phenotype’’) which Dr. Jean-Philippe Coppé et al refer to as the “dark side of tumor suppression”. This is because although cellular senescence is tumor-suppressive mechanism that permanently arrests cells at risk to become malignant, such cells also can have deleterious effects on the tissue microenvironment, which become increasingly detrimental as we age. (23)
Certain proteins secreted by senescent cells (like p16 and p19) suppress tumors, but also correlate with the chronological age of essentially all tissues analyzed both in mice and humans. There may be no other gene or protein whose expression is so robustly correlated with chronological aging across tissues and across species. Further, this is genetically linked to the highest number of age-associated pathologies, including several types of cardiovascular diseases, diabetes, glaucoma, and Alzheimer’s disease. (24)
Clearly, senescent cells accumulate with time and age, and promote age-related disease. Scientists have found that eliminating senescent cells in old mice seems to reverse some of the effects of aging. And the opposite is also true — when senescent cells were injected into young mice, they had debilitating and inflammatory effects, and were harmful to overall health. (25)
What You Can Do
Scientists have made tremendous progress in developing senolytics that clear senescent cells from the body My report on senolytics discusses the efficacy of several compounds geroscientists have discovered that clear senescent cells and slow the aging process. Several of these compounds are slated for clinical trials. (26)
Three senolytics widely available are: Quercetin, Piperlongumine and Fistein. Fistein may be the most potent of the three. (27)
Curcumin is also an excellent inflammation-fighter. Make sure you get a brand that has amendments to improve bioavailability, such as Prohealth’s Optimized Curcumin.
Life Extension offers a promising senolytic-activators, called (what else) Senolytic Activator.
Read my articles:
Can 4 Natural Compounds “Eliminate Senescent Cells” and Prolong Your Healthy Lifespan?
Can New Senolytics Drugs Delay Aging? Part 1: Human Trials Are Coming
Integrative Hallmarks of Aging
The Integrative hallmarks of aging ultimately lead to the functional decline we all see in aging. If geroscientists could stop or impede the Primary and Antagonistic hallmarks of aging, then, in theory, it would have downstream effects in reducing the Integrative hallmarks.
What You Can Do
There are several possibilities for restoring defective intercellular communication underlying aging processes, including genetic, nutritional, or pharmacological interventions that may improve the cell-cell communication properties that are lost with aging, such as gut flora manipulation via probiotic and prebiotic supplementation. (34, 35)
There’s also a new drug called Canakinumab that slashes heart attack and cancer, by dampening inflammaging.
Your Takeaway
I don’t blame you if you scrolled all the way to here to get the bottom line. Surely, there’s a lot to digest here.
The science keeps connecting more dots that helping explain the fundamentals of why and how we age. The best we can do now is to discern the physical and pathological effects stemming from the aging response and seek interventions to slow down or reverse such effects.
With that in mind, remember these four things:
- Fasting is a very potent rejuvination technique. You have some choices here. Intermittent Fasting and Dr. Longo’s Prolon Mimicking Diet can be very helpful at addressing many aspects of aging, as discussed above.
- Senoltyic compounds, such as Quercetin, Piperlongumine and Fistein have been shown to clear senescent cells from the body. Read my article on the topic.
- Inflammation — or as geroscientists now call it, inflammaging — is a big, nasty part of aging, either as an instigator of various degenerative diseases or and amplifier of them. Reduce the chronic inflammation in your body by eating more plants, less meat and dairy; and take curcumin and high quality probiotics and prebiotics, such as those shown above.
- Stem cells may be rejuvinated by a combination of nutracueticals; namely, green tea, astragalus, goji berry extracts, ellagic acid, and vitamin D fermented with a probiotic Lactobacillus strain, which are the ingredients of a brand called Stem-Kine.
Read Part 1
Last Updated on February 7, 2024 by Joe Garma