How To Induce Autophagy Through Four Longevity Pathways

How to induce autophagy is something you need to learn if you’re interested in maximizing longevity and healthspan. The autophagic process is necessary for cells to self-regulate and maintain homeostasis, and you can help make that happen.
Everybody interested in longevity needs to learn how to induce autophagy. This happens through four so-called longevity pathways that act upon several of the Hallmarks of Aging.
I’ll tell you right up front that autophagy is stimulated by exercise, fasting and a few common drugs. But the details are important, the first being, What is autophagy and why should you care about it?
To answer that question, I’m going to summarize an important scientific paper entitled, Maximizing Longevity and Healthspan: Multiple Approaches All Converging on Autophagy. This paper provides an overview of the key longevity signaling pathways associated with aging, the modulation of which has been shown to extend lifespan in a range of model organisms studied.
These pathways converge onto autophagy, a catabolic process that recycles dysfunctional cellular material and maintains energy homeostasis. Along the way as I describe how autophagy happens, I will discuss how established anti-aging approaches including exercise, caloric restriction, and pharmaceutical therapeutics affect these pathways to regulate autophagy in ways that are geroprotective and possibly longevity-enhancing.
Content
Let’s dive in…
What is Autophagy and Why it’s Important?

Autophagy (from the Greek for “self-eating”) is the mechanism by which cells break down and recycle cellular content.
The molecular signatures of aging are being uncovered at a prodigious pace. One thing that’s becoming clear is the remarkable evolutionary conservation of cell signaling pathways across various invertebrate and vertebrate species, meaning from worms to flies to mice, and even to humans.
Autophagy is a cellular process that has been discovered as a nexus to which these pathways converge; a process that removes unnecessary or dysfunctional components of a cell that allows the orderly degradation and recycling of cellular components. Autophagy is catabolic: It induces the cell to eliminate unnecessary cellular components to maintain energy homeostasis and prevent the build-up of toxic material.
There are three forms of autophagy:
- Macroautophagy,
- Microautophagy, and
- Chaperone-mediated autophagy.
This review focuses on macroautophagy, which I’ll simply refer to as “autophagy”.
Check out Dr. Eric Berg’s video that explores the benefits of autophagy:
OK, with that as a primer, let’s move on to understanding the connection between autophagy and longevity, the cellular pathways that suppress or ignite it, and what you can do to regulate those pathways.
Autophagy is Necessary for Longevity and is Associated with the Hallmarks of Aging
It’s been shown that as animals age autophagic activity declines. This has been demonstrated in various animal models, such as worms [1] and mammals, such as mice [2].
One approach to show that autophagy is necessary to confer longevity on those animals studied is to inhibit autophagy in long-lived mutants of a particular animal model and see what happens. Dr. Beth Levine’s group did just that with worms and discovered that inhibiting autophagy in the long-lived mutants nullified the longevity-promoting effect of the mutation [3].
Going in the opposite direction, to demonstrate a causal relationship between autophagy and longevity, some researchers have evaluated the effects of overexpressing autophagy genes rather than inhibiting them. A positive relationship between autophagic activity and lifespan was first shown in flies, resulting in both an increase in lifespan and a reduction in the accumulation of toxic protein aggregates in neurons [4]. This was true for mice as well [5, 6].
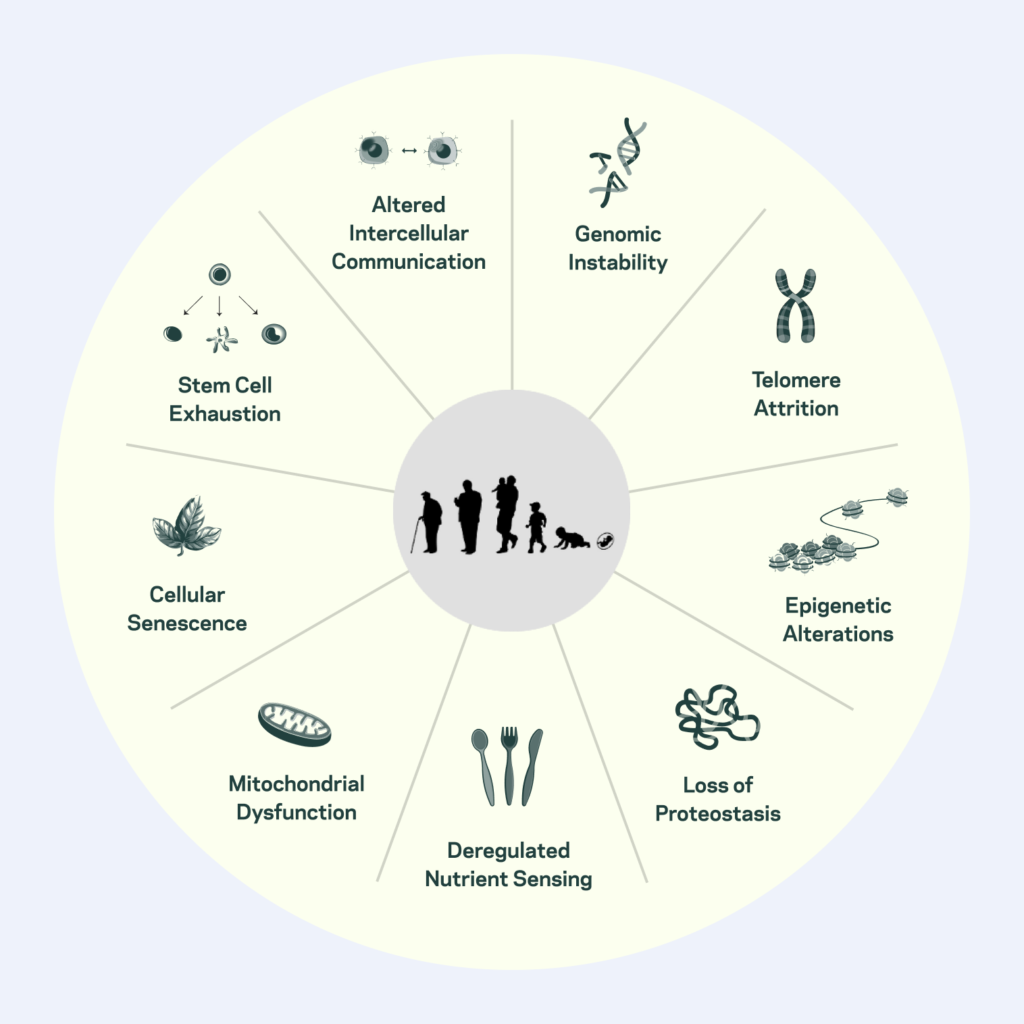
Another substantial indication that autophagy is necessary for longevity is its involvement in most of the nine Hallmarks of Aging. You can delve into these hallmarks in my posts, Can Aging Hallmarks Predict Age-related Disease? and The 4 Primary Reasons We Age and What To Do About It. Suffice to say here that the nine Hallmarks of Aging are genomic instability, telomere shortening, epigenetic alterations, loss of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, cell senescence, stem cell loss and altered intercellular communication [7].
Let’s briefly take a look at how autophagy interacts with a few of the Hallmarks.
The genome is an organism’s complete set of genetic instructions, and the Hallmark “genomic instability” generally refers to the appearance of high frequency of mutations in a single genome, which, incidentally, is a characteristic of almost all human cancers. Autophagy can mitigate the effects of genomic instability by reducing the production of DNA-damaging reactive oxygen species (ROS) production (think free radicals), and by promoting the recycling of DNA repair proteins [8]. Obviously, then, that autophagy can reduce genomic instability is a good thing.
Telomeres are sequences of repetitive DNA that serve to protectively cap the ends of the chromosomes. (Think of them like the plastic tips of a shoelace that protects it from fraying.) Like the rest of a chromosome, including its genes, telomeres are stretches of chemical DNA code.
Telomeres function to protect the ends of chromosomes from sticking to each other. They also protect genetic information during cell division because a short piece of each chromosome is lost every time DNA is replicated.
Although autophagy is unable to revert or stall the Hallmark “telomere attrition”, recent work has shown that telomere dysfunction directly stimulates autophagy to promote the death of precancerous cells [9]. This is a very important function of autophagy, because if nothing abates cancer cell growth, they can threaten the life of the host.
Autophagy has canonical roles (established pathways with common or standard features) in:
- Maintaining proteostasis (the protein complex), nutrient sensing (a key regulator of tissue growth);
- Mitochondrial health (the cell organelles that make ATP, the cells’ energy) via mitophagy (a selective form of macro-autophagy in which dysfunctional mitochondria are specifically targeted for autophagic degradation);
- Maintaining proper immune function (a key component of intercellular communication) by preserving phagocytic activity (the action of a type of cell that has the ability to ingest, and sometimes digest, foreign particles, such as pathogens), and controlling levels of inflammation; and
- the maintenance of stem cells.
The bottom line here is that since 2013 the Hallmarks of Aging have reliably been the reference point for nine characteristics (hallmarks) that help describe aging, and autophagy has been shown to counter the effects of the majority of these Hallmarks.
How To Induce Autophagy Via Longevity Pathways
The Hallmarks of Aging describe what factors constitute aging, but how do they actually happen? In other words, what are the cellular signalling pathways that, perhaps like electricity as an analogy, animate them?
Four well-studied pathways that are known to regulate aging, and whose modulation has been shown to influence the rate of aging are:
- Insulin/IGF-1,
- Mechanistic target of rapamycin (mTOR),
- AMP-activating protein kinase (AMPK), and
- Sirtuin pathways.
Let’s take a look at the relationship between each of these pathways and longevity, their effects on autophagy, and the effects of aging and exercise on these pathways relative to autophagy.
You will find that longevity is increased when Insulin/IGF-1 and mTOR is suppressed and AMPK and Sirtuin pathways are enhanced.
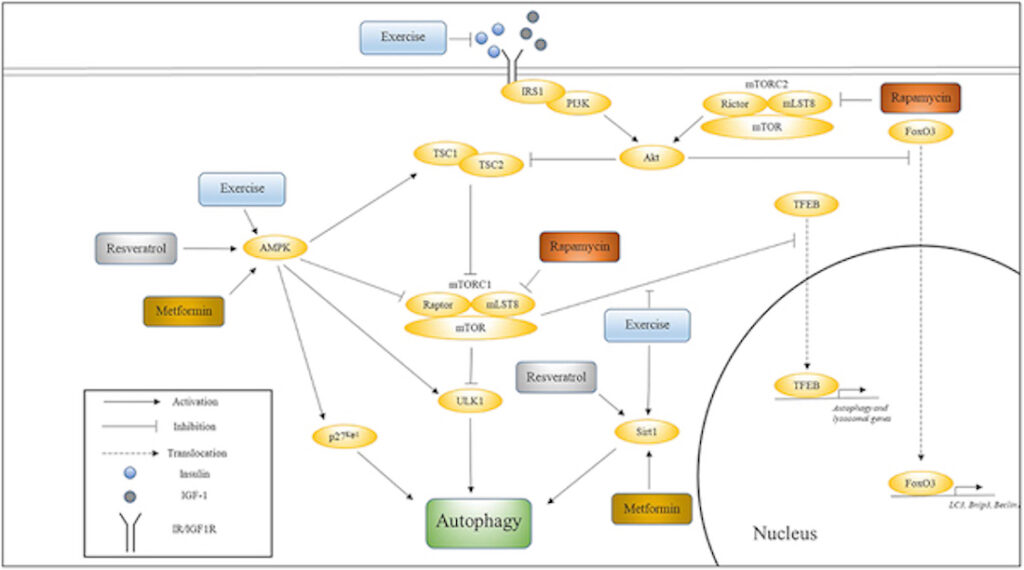
The diagram below illustrates how these various pathways converge onto and activate autophagy. Notice the main instigator – exercise!
The influence of exercise on cell signaling pathways that regulate autophagy
The diagram above shows how various pathways associated with longevity converge onto autophagy, and how exercise influences these pathways. Also indicated are the nodes upon which Metformin, Rapamycin, and Resveratrol are thought to act. I’ll be explaining much of this as we proceed.
As you can observe from the diagram, it all begins with exercise. I pound the drum quite a bit about exercise. I don’t have the exact quote handy, but famed longevity expert Dr. Peter Attia stresses that everything else you do to extend healthspan/lifespan is incidental if exercise isn’t the main component. Two ways to gauge your exercise effort and capacity is via METs and VO2 max, which I describe in the post, How To Reduce Obesity In Older Adults and Improve VO2 Max.
OK, now that the exercise drum has once again been beat, on to the four longevity pathways.
The study I’m summarizing here maps each pathway by its effect on longevity and age, and by the effect of age and exercise on each pathway:
- Pathway x -> Longevity and Autophagy
- Age and Exercise -> Pathway x
First up is insulin/IGF-1 signaling (IIS), its effect on longevity and autophagy, and the effects of age and exercise on IIS.
(1) Insulin/IGF-1 Signaling (IIS)
IIS and Longevity
Insulin is the hormone your pancreas produces to shuttle blood glucose into cells where it is then used for energy to keep your body alive, or stored in the liver, muscles or adipose tissue (body fat) for future use should your fridge ever be empty. Insulin Growth Factor-1 (IGF-1) is a hormone found naturally in your blood. Its main job is to regulate the effects of growth hormone in your body. Together, they are referred to as IIS, and was the first pathway to be shown to affect aging [10].
There’s an inverse relationship between IIS and longevity; meaning that when it comes to longevity, less ISS is better when you’re older. In worms (C. elegans), a loss-of-function mutation in their daf-2 gene responsible for IIS was responsible for more than a two-fold increase in lifespan compared to wild-type (regular) worms. Inhibition of this pathway in vertebrate animal models has also been shown to extend lifespan, but to a lesser degree and inconsistently; nonetheless, the current thinking is that deregulation of IIS confers longevity to some degree in all animal models tested.
Effects of IIS on Autophagy
The C. elegans daf-2 mutants exhibit a pronounced increase in autophagic activity compared to the regular worms, which suggests that the IIS pathway suppresses autophagy. Remember, what makes these worms “mutants” is that their IIS pathway was rendered dysfunctional, which then instigated autophagy. Under conditions of nutrient deprivation, which suppresses IIS, autophagy is upregulated by the expression of autophagy-related genes.
Effects of Age on IIS
When humans and some mammals age they experience a decline in circulating growth hormone (GH) and IGF-1 levels. Centenarians in particular have been shown to have significantly lower levels of circulating IGF-1, and their offspring tend to have both lower levels of circulating IGF-1 and lower IGF-1 activity compared to those whose parents both died relatively young. A recent study also showed a general increase in the expression of genes in the autophagy-lysosomal pathway in centenarians [11].
The autophagy lysosomal pathway is a major mechanism for degrading intracellular macromolecules, which means that very large molecules important to biophysical processes, such as a protein or nucleic acid, are broken down. This intercellular degradation is catabolic, meaning that they’re broken down, and such catabolic products can then be used by the cell for energy, or as building blocks to make other macromolecules.
Unlike IGF-1, circulating insulin levels generally increase with age; in fact, aging is associated with hyperinsulinemia (too much insulin) and insulin resistance (which means the pancreas has to produce excessive amounts of insulin) compared to younger individuals. In contrast, centenarians have been shown to exhibit both a lower degree of insulin resistance and preserved β-cell function. (β-cells are pancreatic cells that make insulin.)
The bottom line here is that aging is associated with decreasing levels of autophagic activity that are partially the result of dysregulated IIS. One way this is evident in humans is that healthy centenarians that don’t fully experience the typical effects of normal aging display both enhanced autophagy and better-preserved and regulated IIS.
Effects of Exercise on IIS
The study here under review cites several other studies to support the strong evidence to suggest that exercise promotes both healthspan and lifespan in worms, flies and mammals. There is also extensive evidence that indicates that exercise effectively suppresses insulin resistance and hyperinsulinemia.
Exercise has been shown to:
- Reduce the incidence of type 2 diabetes (both regular aerobic and resistance exercise).
- Decrease plasma insulin concentration and increase insulin sensitivity in subjects in their sixties.
- improve insulin resistance in elderly women aged 70–80 years (this from an exercise regimen of 20 minutes of resistance band exercise and 30 minutes of walking three times a week for 12 weeks).
- Promote systemic autophagy.
One way exercise increases insulin sensitivity is via contraction-stimulated glucose uptake, which involves the activation of AMPK, a longevity pathway covered below. It’s thought that acute exercise counteracts the autophagy-suppressing effects of IIS through the activation of autophagy promoters (such as AMPK), and maintains long-term autophagic activity via preservation of insulin sensitivity, which enables a reduction in circulating insulin levels.
mTOR 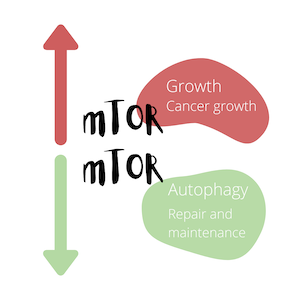
mTOR and Longevity
Like with the IIS pathway, inhibiting mTOR increases longevity. And as with the previously-described daf-2 mutants, C. elegans deficient in mTOR displayed a doubling in lifespan. Mice whose mTOR was suppressed to approximately 25% of normal had significantly extended median lifespans.
Effects of mTOR on Autophagy
Again, as in the case of the daf-2 mutants, inactivation of mTOR signaling in C. elegans resulted in increased levels of autophagy, and when autophagy was suppressed, these lifespan-increasing effects were reversed.
Effects of Age on mTOR
Full activation of mTORC1 (there’s also a complex called mTOR2) requires both growth factors (such as insulin or IGF-1), and a supply of amino acids (such as leucine, methionine, and arginine). These growth factors are, by definition, anabolic, which means they facilitate the growth and development of an organism.
Anabolic factors, including hyperinsulinemia, methionine (an amino acid) and homocysteine (related to the amino acid cysteine) increase with age and upregulate mTOR, suggesting that mTORC1 activity also increases with age. Increased mTORC1 activity would therefore serve as another reason for the general decline in autophagic activity seen with age.
Effects of Exercise on mTOR
Exercise has been shown to inhibit the mTORC1 pathway, which is yet another lifespan-extending effect of exercise attributable to longevity pathways.
AMPK 
AMPK and Longevity
A positive correlation between AMPK activity and longevity has been demonstrated in both invertebrate and vertebrate models. C. elegans worms that lack AMPK experienced a 12% reduction in lifespan compared to wild-type worms, whereas, AMPK overexpression resulted in a 13% increase in lifespan. Female mice chronically treated with Metformin, an anti-diabetic drug that activates AMPK, experienced maximum lifespan increase of approximately 10% compared to control mice .
Effects of AMPK on Autophagy
AMPK is a potent promoter of autophagy. Under conditions of stress, such as low nutrient availability, particularly glucose, AMPK has been shown to promote autophagy.
AMPK also promotes autophagy by directly and indirectly inhibiting mTORC1.
Effects of Age on AMPK
Aging has a strong inhibitory effect on AMPK activity. Whether young or old, baseline AMPK activity in rats was comparable, but old rats have a severely compromised ability to respond to activators of AMPK, such as Acetyl-L-Carnitine, or exercise.
Effects of Exercise on AMPK
Various studies have demonstrated the ability of exercise to promote AMPK activation [12], but exercise has been shown to be unable to activate AMPK in aged tissue; therefore, ideally, in order for exercise to have an effect on autophagy via this pathway, it should be initiated early in life. That said, studies that indicate this limitation subjected their rats to only five days of treadmill exercise, suggesting that a longer term exercise regimen might overcome this inability to activate AMPK.
Sirtuins 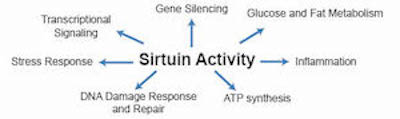
Sirtuins and Longevity
Sirtuins are a type of enzyme that when activated has been linked to lifespan extension in both invertebrates and vertebrates. Increased gene dosage of one type of sirtuin called Sirt2.1 in C. elegans resulted in up to a 50% increase in lifespan.
Effects of Sirtuins on Autophagy
Sirt1 has been shown to play a role in the regulation of and promotion of autophagy via direct interaction with participants in the autophagic pathway, and via the deacetylation of proteins (a protein modification) involved in the autophagy pathway.
Effects of Age on Sirtuins
A general decline in sirtuin function with age has been observed, especially in the liver, heart, kidney and lung of aged rats compared to young controls. (Similarly, a decrease in Sirt1 expression has been detected in the arteries of both mice and humans.) This decline in activity has been attributed to lower levels of NAD+, which is an essential coenzyme in every living cell that plays a critical role in supporting many biological processes.
Effects of Exercise on Sirtuins
Exercise has been shown to be a potent activator of sirtuins. Old rats subjected to an eight-week long regimen of treadmill exercise experienced a significant increase in Sirt1 activity compared to both young and sedentary old rats. This also happened in humans — both old and young human subjects experienced a significant increase in skeletal muscle Sirt1 expression after just one bout of intense treadmill exercise [13].
Exercise, Diet and Pharmacological Agents Activate Autophagy
Many drugs have been identified that can modulate each of the above pathways that activate autophagy. Vakifahmetoglu-Norberg et al., 2015 has such a list in Table 1 of their paper, Pharmacologic agents targeting autophagy
Autophagy-promoting drugs have demonstrated varying levels of success in preclinical settings, but some certain caveats must be noted; for instance:
- They typically target just one protein or pathway, and
- Their potentially negative side-effects have not been thoroughly examined, particularly in the setting of human biology.
So, why mess with drugs when alternative, non-pharmacological approaches such as exercise and calorie restriction (including fasting mimicking diets such as ProLon) have been shown to be potent autophagy activators that effectively target all of the previously described longevity pathways? Among healthy individuals, moderate exercise and carefully monitored caloric restriction have few adverse side effects; therefore, consider exercise and caloric restriction, perhaps via fasting for two days every quarter, or doing a five day ProLon diet as your first steps to upregulate autophagy.
The next step is to monitor the developments of the some of the best-studied autophagy promoting agents:
- Metformin,
- Rapamycin, and
- Resveratrol.
Metformin is a drug that is used to treat type 2 diabetes for longer than 50 years, and has been proven to be safe for most people. Metformin began to be studied as a longevity-promoting drug when doctors realized that type 2 diabetes patients on the drug were out-living non-diabetics. Metformin promotes autophagy by activation of both AMPK and Sirt1.
Resveratrol is a polyphenol compound found in certain plants and in red wine, and also has been shown to activate AMPK and Sirt1. But there’s been some controversy about it — trials in clinical settings have produced unclear and sometimes contradictory findings, as described in Bitterman and Chung (2015) and in a video produced by Dr. Brad Stanfield that I include in my review of the Resveratrol controversy in the post, Does Resveratrol Really Activate Sirtuins?.
Rapamycin is a type of antibiotic that indirectly activates autophagy by inhibiting mTORC1, the pathway that gets it’s name from Rapamycin — mechanistic target of rapamycin. The discovery of Rapamycin is among the most interesting in the drug world, as it was found on Easter Island by microbiologist Georges Nógrády after he explored why the barefoot inhabitants there didn’t get tetanus, a common toxic producing bacterium in that part of the world. It was discovered that a new antifungal compound in soil samples was produced by the bacterium Streptomyces hygroscopicus, later renamed rapamycin in honor of Rap Nui, the traditional name for Easter Island.
Although it’s a potent promoter of autophagy, Rapamycin treatment is associated with various negative side-effects, including immunosuppression and the potential induction of insulin resistance via off-target inhibition of mTORC2. What this means is that few doctors will prescribe it for you for lifespan enhancements. You risk your health if you get it yourself, say, from some out-of-country online pharmacy.
In summary, although these and various other drugs have been shown to induce autophagy and even have life-extending effects in animal models, there are concerns about their use in humans, so rather than take these drugs, try fasting every once in a while and exercise with vigor, regularly.
Your Takeaway on How to Induce Autophagy
Autophagy plays a pivotal role in healthspan and lifespan extension. Four particular cell signaling pathways (Insulin/IGF-1, mTOR, AMPK and Sirtuins) have beneficial effects on longevity, and autophagy is a necessary mediator of these effects.
How to induce autophagy in your own body can be done by consistent exercise, periodic fasting and/or the Prolon Fasting Mimicking Diet, and several pharmaceutical therapies.
Do not take rapamycin without the guidance of your doctor. It is typically used to reduce the body’s immunity during organ transplant so the transplanted organ is not rejected by the body. You do not want to risk impairing your immune system by taking the wrong amount of or wrong dose frequency of rapamycin.
Last Updated on February 7, 2024 by Joe Garma