The Four Horsemen of Disease: The Impact of Aging Hallmarks and Signaling Dysfunction, Part 3

The Four Horsemen of Disease is a colloquialism for the most pernicious diseases that knock us to our knees us as we age. We need not get trampled. Here’s how chronic disease is linked to aging hallmarks and cellular signaling pathways, and what to do about it.
The “Four Horsemen of Disease” refer to the four diseases that most of us get as we get older, and for many, many of us, are the cause of our death.
If you read Part 1 of this three-part series, you learned that the so-called The Hallmarks of Aging encompass a comprehensive set of biological processes that significantly contribute to the aging process.
In Part 2, you learned that these Hallmarks are interlinked through cellular signaling pathways. When these aging hallmarks remain unchanged and cellular communication is disrupted, it can result in the very common age-related diseases that ultimately kill us.
Now, here in Part 3, you will see how the major diseases of aging and death (the Four Horsemen), The Hallmarks of Aging and cellular signaling pathways link together to age and eventual take us to our demise. Moreover, I’ll tell you about some basic things you can do to push back against the tide.
Let’s begin with the diseases of aging. Which diseases am I referring to?
The Four Horsemen of Disease
Well, how about the six leading causes of death in the US, Canada, and Europe [1][2][3][4][5]. (Similar statistics apply to most other industrialized nations.)
Leading Causes of Death
| Rank | United States | Canada | Europe |
| #1 | Heart Disease | Cancer | Cancer |
| #2 | Cancer | Heart Disease | Heart Disease |
| #3 | Stroke | Stroke | Stroke |
| #4 | Chronic Lower Respiratory Diseases | Chronic Lower Respiratory Diseases | Respiratory Diseases |
| #5 | Alzheimer’s Disease and Dementia | Alzheimer’s Disease and Dementia | Alzheimer’s Disease and Dementia |
| #6 | Diabetes | Diabetes | Liver Disease |
Four of those chronic diseases related to aging are sometimes referred to as the Four Horsemen of Disease, a colloquial or metaphorical way of referring to some of the most common and significant health challenges people face, and a clever takeoff from the Four Horsemen of the Apocalypse from the Book of Revelation in the New Testament of the Bible.
There’s a famous painting of this by Russian painter Viktor Vasnetsov [vas_nits_ov]. From left to right are the depictions of Death, Famine, War, and Conquest.
And just as death, famine, war, and conquest are apocalyptic, so are diseases that replace them in the Four Horsemen of Disease.
The Four Horsemen of Disease
|
These chronic diseases are the four most common and deadly age-related diseases that are responsible for the great majority of deaths in people over 50 in industrialized nations who do not smoke [6][7]. But you might wonder, Why do the “Horsemen” include these four among the six top killers, excluding stroke and respiratory diseases that kill more people than neurodegenerative diseases and metabolic-based diseases?
There’s a three-fold answer to that question:
- The four diseases of the “Four Horsemen” make that list because of how many people they kill, their high prevalence, their impact on quality of life, and their economic burden on healthcare systems.
- Stroke can be included as an atherosclerotic disease, because most strokes happen due to atherosclerosis.
- Metabolic disorders is the instigator of, or is associated with, a wide variety of diseases that beset the elderly.
Let’s briefly examine each of these: The burden, the connection between stroke and atherosclerotic disease, and the interconnecting web — metabolic disorders. And then I’m going to throw a curveball at you.
Disease Burden
The inclusion of neurodegenerative diseases like Alzheimer’s and dementia and Metabolic disorders, such as type 2 diabetes and obesity in discussions of the “Four Horsemen of Disease” is often based on their significant impact on public health and overall healthcare systems, rather than just their mortality rates.
While it’s true that diseases like stroke and respiratory diseases may cause relatively more immediate deaths, neurodegenerative and metabolic diseases have five distinct characteristics that make them salient in public health discussions:
- Chronic Nature and Long-Term Impact: neurodegenerative disease and Metabolic disorders are chronic conditions that can affect individuals over many years or even decades. They have a long-term impact on individuals’ quality of life, daily functioning, and overall well-being.
- Economic Burden: Both neurodegenerative disease and Metabolic disorders impose a substantial economic burden on healthcare systems and society as a whole. The cost of managing and treating these conditions, including long-term care and medications, can be significant.
- Prevalence: Neurodegenerative disease and diabetes are prevalent in aging populations, and their prevalence is expected to increase as the population ages. This makes them important public health challenges.
- Complications: Both neurodegenerative disease and diabetes can lead to severe complications and comorbidities, which can contribute to morbidity and mortality beyond the diseases themselves. For example, diabetes can lead to heart disease, kidney disease, and neuropathy.
- Quality of Life: These conditions significantly impact the quality of life of affected individuals and their caregivers, which has a broader societal impact.
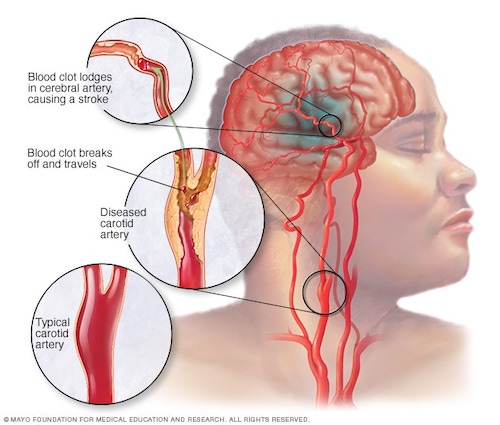
Now, if you’ve ever had a stroke or know someone who has, you might think that it meets the five characteristics I just went over, but a discussion over this is moot, because the most common type of stroke by far is an atherosclerotic disease itself, thus belonging to the first of the Horsemen of Disease, atherosclerosis.
Stroke
The most common type of stroke is an ischemic stroke, which covers 85% of the cases produced by a blockage of blood vessels. The other less common type which covers about 15% of cases of stroke is caused by bleeding in or around the brain which is called a hemorrhagic stroke [8].
Most ischemic strokes are caused by atherosclerotic disease, and so, as I said, it’s a type of atherosclerotic disease included in the Four Horsemen.
Atherosclerosis is the buildup of fatty substances in the arteries, leading to the narrowing of the arteries and reducing blood flow to the brain. It’s a slow, complex disease that typically starts in childhood and progresses with age. The narrowing of the arteries can cause a blood clot to form, which can block blood flow to the brain and cause an ischemic stroke.
Metabolic disorders
Although it encompasses a broader array of metabolic dysfunction, “metabolic disorders” is often referred to as “metabolic syndrome”, which is an instigator of several chronic diseases, including heart disease, stroke, chronic respiratory diseases, and diabetes [9][10][11].
Metabolic syndrome is a clearly defined group of metabolic disorders represented as a cluster of conditions that occur together, including increased blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol or triglyceride levels.
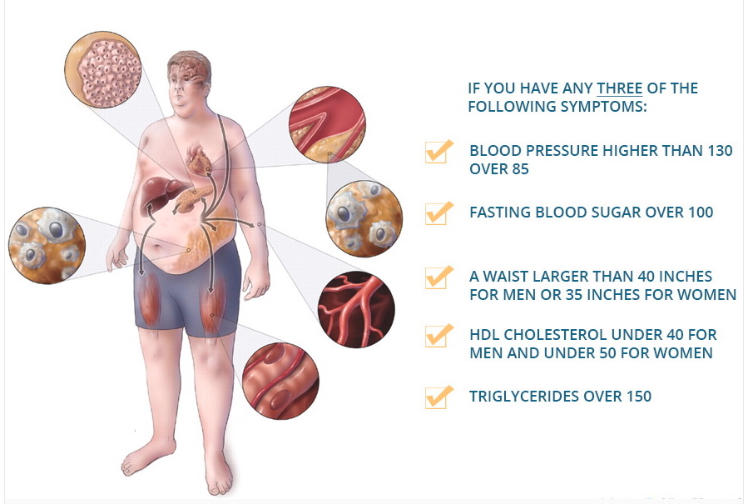 Having three or more of the risk factors shown in the above image increases the risk of developing type 2 diabetes and heart disease [12] There’s also evidence to suggest that metabolic diseases such as diabetes may contribute to the development of Alzheimer’s disease [13][14].
Having three or more of the risk factors shown in the above image increases the risk of developing type 2 diabetes and heart disease [12] There’s also evidence to suggest that metabolic diseases such as diabetes may contribute to the development of Alzheimer’s disease [13][14].
Once you have metabolic syndrome you’re at an increased risk for many diseases. Your risk of cardiovascular disease goes up by 135 percent; cardiovascular mortality goes up by 140 percent; all-cause mortality goes up by 58 percent; heart attack risk increases by 99 percent; the risk of stroke climbs to 127 percent. With cancer, metabolic syndrome increases the age-adjusted risk of cancer mortality by 56 percent. There’s also a handful of cancers that seem especially impacted by metabolic syndrome, such as a seven times greater risk for endometrial cancer (the inner lining of the uterus), nearly a five times greater risk for gastric (stomach) cancer, and the risk for liver kidney is twice as likely [15].
Metabolic Syndrome Increases Disease Risk
- All-cause mortality ↑58%
- Cardiovascular mortality ↑140%
- Cardiovascular disease ↑135%
- Heart attack ↑99%
- Stroke ↑127%
- Cancer mortality ↑56%
- Endometrial cancer ↑7x
- Gastric cancer ↑5x
- Liver cancer ↑2x
So, should you be worried about any of this?
The statistics suggest you should at least find out where you stand relative to all of these Four Horsemen of Disease. For instance, there’s between 100 and 125 million people in the United States that have metabolic syndrome, and, interestingly, a third of these folks are not obese, which is typically a signal of concern [16].
Again, astonishingly, between 100 and 125 million people in the U.S. have three or more of the five conditions that indicate metabolic syndrome.
Metabolic Syndrome: 3+ of these 5:
- Blood pressure above 130 over 85
- Fasting blood sugar over 100 mg/dL (5.6 mmol/L)
- A waist circumference over 40 inches (101.6 cm) for men, 35 inches (88.9 cm) for women
- HDL under 40 mg/dL (2.2 mmol/L) for men, 35 mg/dL (1.9 mmol/L) for women
- Triglycerides over 150 mg/dL (8.3 mmol/L).
And there’s one more thing about metabolic syndrome pertinent to this discussion. That’s the curveball.
The Curveball -> Inflammation
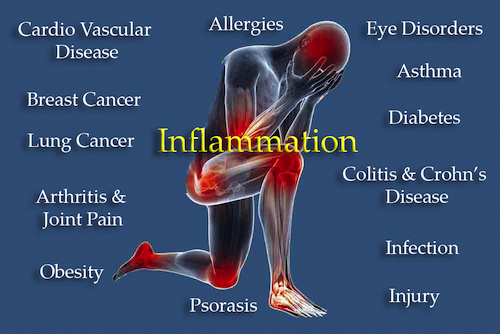
If you had to choose, you could reliably assert that chronic/systemic inflammation (inflammaging) may be the most significant precursor to many of the chronic diseases associated with aging, including the Four Horsemen of Disease.
Chronic inflammation is characterized by the presence of immune cells and inflammatory molecules that persist over time, leading to tissue damage and dysfunction.
The aging hallmark termed “Chronic/Systemic Inflammation” is interrelated to other Hallmarks of Aging presented in Part 1. Metabolic disorders, for instance, can contribute to the development of atherosclerotic cardiovascular disease, cancer, insulin resistance, and diabetes, as well as vascular and neurological complications because of its association with chronic inflammation [16]. Chronic inflammation is a common factor in those diseases, as well as cancer, stroke, high blood pressure, and respiratory diseases [17].
The Links Between Disease, Aging Hallmarks and Signaling Pathways
Chronic inflammation is significantly interrelated to the other aging hallmarks as well [18][19]; for example:
- Genomic instability — Chronic inflammation can contribute to genomic instability by promoting DNA damage and mutations.
- Telomere attrition –Chronic inflammation can accelerate telomere shortening, which is associated with cellular senescence and aging-related diseases.
- Epigenetic alterations — Chronic inflammation can alter the epigenetic landscape of cells, leading to changes in gene expression and cellular function.
- Loss of proteostasis — Chronic inflammation can disrupt protein homeostasis, leading to the accumulation of misfolded and damaged proteins that can contribute to the development of age-related diseases.
- Deregulated nutrient-sensing — Chronic inflammation can disrupt nutrient sensing pathways, leading to metabolic derangements such as insulin resistance and type 2 diabetes.
- Mitochondrial dysfunction — Chronic inflammation can impair mitochondrial function, leading to the production of reactive oxygen species and cellular damage.
- Cellular senescence — Chronic inflammation can promote cellular senescence, which is a hallmark of aging that is associated with tissue dysfunction and the development of age-related diseases.
As reviewed in Part 2, the cellular signaling pathways that are relevant to this interconnection between chronic inflammation and other aging hallmarks include [18][20][21]:
- NF-κB, a transcription factor that plays a central role in the regulation of inflammation and immune responses. (Transcription factors are proteins involved in the process of converting, or transcribing, DNA into RNA.)
- mTOR, a special enzyme (protein kinase) involved in the regulation of cellular metabolism and growth, is often dysregulated (not properly functioning) in cancer and metabolic diseases such as diabetes.
- AMPK, another special enzyme (AMP-activated protein kinase) that is involved in the regulation of cellular metabolism and energy homeostasis, and it is often dysregulated in metabolic diseases such as diabetes.
Let’s summarize all this in a handy table I put together for your enduring delight.
The table below shows the associations between the Hallmarks of Aging, the cellular signaling pathways and the geroprotectors that modulate them in order to extend health span, and possibly lifespan as well. (Geroprotectors are typically defined as substances that aim to affect the root cause of aging and age-related diseases, and thus prolong the healthspan/lifespan, but I also include behaviors that have geroprotective effects, such as exercise and diet.)
| Hallmark of Aging | Cellular Signaling Pathway Modulation | Geroprotectors
|
| — Atherosclerosis — | ||
| 6 Hallmarks | 6 Pathways | Geroprotectors |
| Telomere Attrition | ↓mTOR
↑AMPK ↑FOXO |
Caloric Restriction, Exercise, Rapamycin, Stress Mgt, Sleep |
| Mitochondrial Dysfunction | ↑Sirtuins | Diet, Exercise, Resveratrol*, NAD+ Precursors |
| Cellular Senescence | ↑FOXO ↑p53 | Exercise, Caloric Restriction, Stress Mgt., Social Engagement, Rapamycin, Metformin* |
| Stem Cell Exhaustion | ↑FOXO | Exercise, Caloric Restriction, Stress Mgt., Social Engagement, Metformin* |
| Altered Intercellular Communication | ↓NF-kB | Exercise, Caloric Restriction, Stress Mgt., Sleep, Social Engagement, Resveratrol* |
| Chronic/Systemic Inflammation | ↓NF-kB | Exercise, Diet, Caloric Restriction, Stress Mgt.Resveratrol*, Metformin*, Curcumin |
| — Cancer — | ||
| 6 Hallmarks | 6 Pathways | Geroprotectors |
| Genomic Instability | ↓mTOR ↑FOXO | Caloric Restriction, Diet, Rapamycin, Metformin*, Stress Mgt. |
| Epigenetic Alterations | ↑Sirtuins | Resveratrol, NAD+ Precursors, Stress Mgt., Sleep |
| Cellular Senescence | ↑FOXO ↑p53 | Exercise, Caloric Restriction, Stress Mgt., Social Engagement, Rapamycin, Metformin* |
| Stem Cell Exhaustion | ↑FOXO | Exercise, Caloric Restriction, Stress Mgt., Social Engagement, Metformin* |
| Chronic/Systemic Inflammation | ↓NF-kB | Exercise, Diet, Caloric Restriction, Curcumin, Resveratrol*, Metformin* |
| Dysregulation of RNA Processing/Splicing Deregulation | ↑Sirtuins | Resveratrol*, NAD+ Precursors |
| — Neurodegenerative Diseases — | ||
| 5 Hallmarks | 4 Pathways | Geroprotectors |
| Loss of Proteostasis | ↑FOXO | Exercise, Caloric Restriction, Metformin* |
| Mitochondrial Dysfunction | ↑Sirtuins | Diet, Exercise, Resveratrol*, NAD+ Precursors |
| Compromised/Disabled Macroautophagy | ↑AMPK | Exercise, Caloric Restriction, Metformin*, Sleep |
| Chronic/Systemic Inflammation | ↓NF-kB | Exercise, Diet, Caloric Restriction, Curcumin, Resveratrol*, Metformin* |
| Dysregulation of RNA Processing/Splicing Deregulation | ↑Sirtuins | Resveratrol*, NAD+ Precursors |
| — Metabolic disorders — | ||
| 5 Hallmark | 4 Pathways | Geroprotectors |
| Deregulated Nutrient Sensing | ↓IGF-1
↑AMPK |
Diet, Caloric Restriction, Diet Metformin* |
| Mitochondrial Dysfunction | ↑Sirtuins | Diet, Exercise, Resveratrol*, NAD+ Precursors |
| Compromised/Disabled Macroautophagy | ↑AMPK | Exercise, Caloric Restriction, Metformin*, Sleep |
| Chronic/Systemic Inflammation | ↓NF-kB | Exercise, Caloric Restriction, Curcumin, Resveratrol*, Metformin* |
| Microbiome Disturbance/Dysbiosis | None directly | Probiotics, Prebiotics, Dietary Fiber |
*Recent studies have contradicted earlier studies about the geroprotective benefits of metformin and resveratrol. I have updates for metformin here, and resveratrol here. The bottom line: I would not use them presently as geroprotectors; however, metformin is widely prescribed for type 2 diabetes.
There are two big takeaway from the above table that I’d like to underscore:
- Chronic/Systemic Inflammation (4) and Mitochondrial Dysfunction (3) are the aging hallmarks most associated with diseases of aging; respectively, four and three out of the four diseases here profiled.
- Exercise, diet and caloric restriction are three prominent geroprotectors that have the potential to potential to mitigate many of the Hallmarks of Aging — including chronic inflammation and mitochondrial impairment — and modulate signaling pathways, thereby influencing the rate and quality of the aging process.
Now, to let you quickly see the effects of some of the most impactful geroprotectors on aging hallmarks and signaling pathways, let’s refashion how the material is presented:
| — Diet and Nutrition — | |
| Effects on Hallmarks | Effect on Signaling Pathways |
| Poor diet can contribute to genomic instability, deregulated nutrient sensing, mitochondrial dysfunction, and chronic inflammation. It may also affect the microbiome and lead to dysbiosis. | Nutrient-rich diets can influence mTOR, AMPK, and sirtuin pathways, affecting metabolism and longevity. Inflammatory diets can activate NF-kB, contributing to systemic inflammation. The gut microbiota can influence NF-kB and AMPK pathways, impacting inflammation and metabolism. |
| — Exercise — | |
| Effects on Hallmarks | Effect on Signaling Pathways |
| Regular exercise can impact telomere attrition, mitochondrial function, cellular senescence, and altered intercellular communication. It may also influence stem cell exhaustion and autophagy. | Exercise activates AMPK, which can promote mitochondrial health and autophagy. It can also affect IGF-1 and FOXO pathways, influencing cellular repair and senescence. |
| — Stress Management — | |
| Effects on Hallmarks | Effect on Signaling Pathways |
| Chronic stress can influence genomic instability, telomere attrition, epigenetic alterations, cellular senescence, and systemic inflammation. It may also lead to altered intercellular communication. | Stress activates p53 and NF-kB pathways, contributing to cellular damage and inflammation. Stress reduction techniques may mitigate these effects. |
| — Sleep Quality — | |
| Effects on Hallmarks | Effect on Signaling Pathways |
| Inadequate sleep can contribute to telomere attrition, epigenetic alterations, compromised autophagy, and altered intercellular communication. | Sleep disruption can influence mTOR and AMPK pathways, affecting cellular metabolism and repair processes. |
| — Social Engagement — | |
| Effects on Hallmarks | Effect on Signaling Pathways |
| Positive social interactions can impact altered intercellular communication, cellular senescence, and stem cell exhaustion. | Social engagement may affect NF-kB and AMPK pathways, influencing inflammation and energy metabolism. |
Here’s what you need to avoid:
| — Smoking and Alcohol Consumption — | |
| Effects on Hallmarks | Effect on Signaling Pathways |
| Smoking and alcohol consumption can lead to genomic instability, compromised autophagy, altered intercellular communication, and chronic inflammation. | Smoking-induced damage can activate p53 and NF-kB pathways, contributing to cellular dysfunction. Alcohol may influence similar pathways. |
| — Environmental Exposures — | |
| Effects on Hallmarks | Effect on Signaling Pathways |
| Environmental toxins can contribute to genomic instability, epigenetic alterations, mitochondrial dysfunction, and systemic inflammation. | Exposure to pollutants can activate p53 and NF-kB pathways, promoting cellular stress responses and inflammation. |
(Sources for the above tables: [21][22][23][24][25][26]).
In summary, diet, exercise, caloric restriction, restorative sleep, stress management, and social interaction can play important, positive roles in promoting healthy aging by influencing aging hallmarks, modulating cellular signaling pathways, and reducing the risk of chronic diseases; whereas smoking, alcohol and toxins and pollutants can have a negative role.
Of course, the details matter. Although the science about how we age may be new to you, the importance of diet, exercise, sleep and many of the rest of the geroprotectors I’ve outlined are well known. And yet there’s often a yawning gap between knowing the generalities of what is good versus the specifics about what to do and how to do them.
That’s one big reason I’m working on an anti-aging course. You may be alerted about when it’s available, as well as get my weekly Newsletter, by becoming a Subscriber.
In the meantime, I have some links to share that can get you started on the road to healthy, enduring healthspan:
Your Takeaway
This three-part series covered a lot of ground.
In Part 1, I identified the biological mechanisms behind why we age, the so-called Hallmarks of Aging. Understanding them is the first step to learning what to do to slow the clock.
Part 2 was about cell chatter, or to put it more scientifically: cellular signaling pathways. It is these “pathways” that interconnect the aging hallmarks, because none of them happen in isolation.
Finally, in Part 3 on this very page, I connected the dots between the aging hallmarks, cellular signaling and the four often deadly chronic diseases that beset so many of us as we age.
Here are the four things to remember from this three-part series:
(1) The Hallmarks of Aging are fundamental biological characteristics and mechanisms associated with the aging process. Knowledge of these hallmarks is essential for understanding aging, age-related diseases, and potential interventions to improve health and longevity in older individuals. (If you’re not one now, you will be, hopefully.) It has far-reaching implications for healthcare, research, and public health as the world’s population continues to age.
(2) Cellular signaling pathways are intricate networks of molecular interactions within cells that regulate various physiological processes and responses to external signals. These pathways transmit information from the cell’s external environment to its interior, allowing cells to coordinate and execute a wide range of functions, including growth, metabolism, immune responses, and cell survival or death. Such pathways are fundamental to the regulation of cellular functions and are intimately connected to the Hallmarks of Aging. Understanding these pathways is crucial for both research into the aging process, and the development of interventions and therapies to promote healthy aging and mitigate age-related diseases.
(3) The age-related diseases reviewed here (atherscelerosis, cancer, neurodegerative diseases and metabolic disorder-based diseases) among others, often share underlying mechanisms and cellular signaling pathways with the Hallmarks of Aging. Dysregulation of these pathways can contribute to disease development and progression. Understanding these interconnections provides insights into the biology of aging, informs therapeutic strategies, and has the potential to enhance both the duration and quality of life in older individuals. (And, dear reader, that’s the goal.)
(4) So far, the nmost significant geroprotectors for potentially slowing down the rate of aging, and mitigating age-associated diseases is to exercise consistently, eat healthily, regularly be in caloric deficit (ie: eat fewer calories than you expend from time to time), get restorative sleep, manage your stress and enjoy social bonding.
That’s it.
If you got something worthwhile from this three-part series, please share it.
Choose to live long and strong.
I’ll show you how!
Last Updated on February 7, 2024 by Joe Garma