The New Hallmarks of Aging: Why You Age, Part 1

The new Hallmarks of Aging take another stride in the direction of identifying all the biological mechanisms behind why we age. Understanding them is the first step to learning what to do to slow the clock.
New Hallmarks of Aging add important biological mechanisms to our understanding about why we age. But why should you care about any of these so-called aging hallmarks?
Well, you may not be interested in being able to recite and explain each one, but these aging hallmarks as a whole are the reasons that we gradually deteriorate, get old and eventually meet our maker, or dust-to-dust, or some such thing.
Why care if there’s nothing you can do about it?
But, au contraire — there’s a lot you can do about it, and that’s the whole point of a three-part series I’m working on, beginning with what you’re now reading.
This Part 1 is my long answer to that question about why you age and what you can do about it:
- In this post, Part 1, you’ll learn about the old and new Hallmarks of Aging;
- Part 2 explores how the cells “speak” to the biological processes that constitute the aging hallmarks, and why they, in effect, start to slur over time, adding to the aging process; and
- In Part 3, I reveal how all of this contributes to the Four Horsemen of Disease that Dr. Peter Attia recently popularized in his book, Outlive. (Subscribe to the Newsletter to be alerted when it’s published.)
Let’s dig in…
How the New Hallmarks of Aging Came to Be
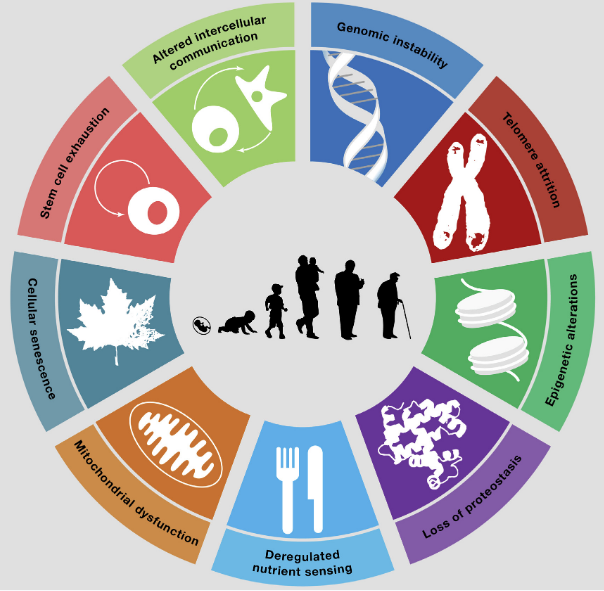
New “Hallmarks of Aging” have been recently added to the original nine established in 2013 when Carlos López-Otín, a Professor in the Biochemistry and Molecular Biology at the University of Ovied, and his associates published a groundbreaking paper about the nine Hallmarks of Aging in an attempt to define the mechanisms of aging.
The Nine Hallmarks of Aging (2013):
- Genomic instability
- Telomere attrition
- Epigenetic alterations
- Loss of proteostasis
- Deregulated nutrient-sensing
- Mitochondrial dysfunction
- Cellular senescence
- Stem cell exhaustion
- Altered intercellular communication
Here’s an illustration of them from that paper:
Since then, these nine hallmarks have become the bedrock understanding about why aging happens, and has been cited by more than 5,000 research papers related to aging…. MORE ON POPULARITY
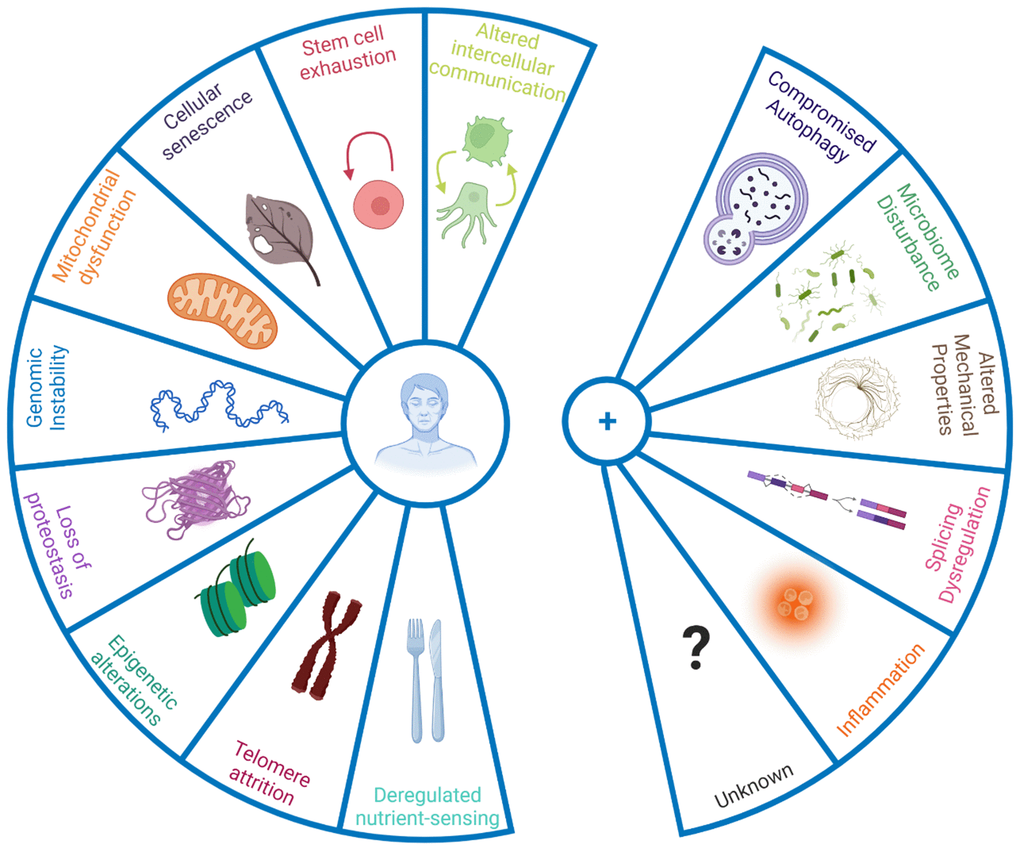
If you google “Hallmarks of Aging”, you’re likely to get results of just nine, the original nine established in 2013, even though several more have been identified since 2013. In 2022, a group of gerontologists met at a conference in Copenhagen and identified five more hallmarks of aging:
Copenhagen’s additional five Hallmarks of Aging
- Compromised autophagy
- Microbiome disturbance
- Altered mechanical properties
- RNA Splicing Dysregulation
- Systemic inflammation
Is your head spinning yet?
Well, take a deep breath, because there’s more.
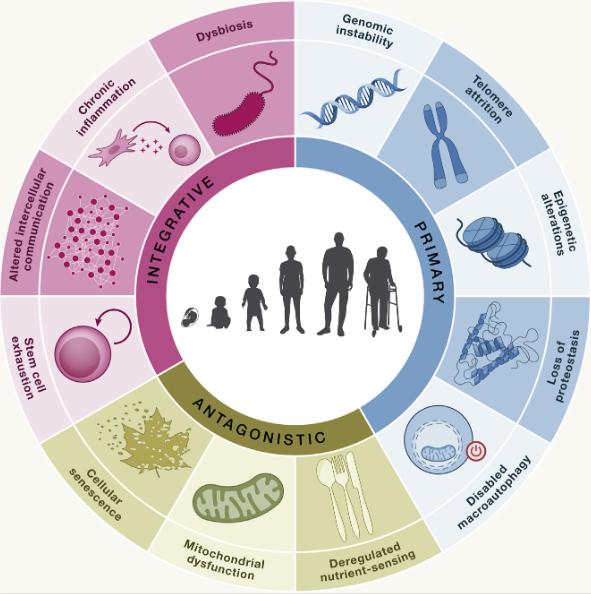
Earlier this year, 2023 — just one year after the Copenhagen meeting — Carlos and his team knocked out three more aging hallmarks that were reviewed in their paper, The Hallmarks of Aging – – An expanding universe.
Carlos López-Otín’s three new Hallmarks of Aging:
- Disabled macroautophagy
- Chronic inflammation
- Dysbiosis
Along with the original nine, they look like this:
There are redundancies in the new Hallmarks of Aging mechanisms that the Carlos López-Otín team and Copenhagen group select as the primary biological processes that are thought to contribute to the aging process (see table below). When you account for similarities, the net new Hallmarks of Aging identified by López-Otín and Copenhagen number 14.
Net Number of New Hallmarks of Aging (14)
| López-Otín Original 9
Genomic instability Telomere attrition Epigenetic alterations Loss of proteostasis Deregulated nutrient-sensing Mitochondrial dysfunction Cellular senescence Stem cell exhaustion Altered intercellular communication López-Otín New 3 Disabled macroautophagy Chronic inflammation Dysbiosis |
Copenhagen Supports Original 9
✔ ✔ ✔ ✔ ✔ ✔ ✔ ✔ ✔ Adds Same 3 (different terminology) ↔ Compromised autophagy ↔ Systemic inflammation ↔ Microbiome disturbance + Net 2 New Added: Altered mechanical properties Dysregulation of RNA processing/RNA Splicing Dysregulation |
And this is well represented graphically by the Copenhagen illustration above.
Now, having identified the old and new Hallmarks of Aging, I want to underscore that none are written in stone. The science of aging is evolving, and new discoveries might change what constitutes aging hallmarks, but to qualify as one, two things must be true:
- The processes identified by an aging Hallmark should change with biological age, not simply in a correlative manner, but have a causal role; and thus,
- Interventions that address the hallmarks should, at the very least, halt further detrimental aspects of aging, and preferably improve phenotypes associated with aging. (A phenotyle is the set of observable characteristics of an individual.)
The simple way to put it is that a Hallmark of Aging causes some aspect of aging, and if that Hallmark is mitigated, we age better, slower, healthier.
And that’s why you should care about them. They give us something to focus on. You should care about the Hallmarks of Aging for three basic reasons:
#1 They help identify the mechanisms of aging which is characterized by a progressive loss of bodily function that leads to increased vulnerability to death.
#2 This deterioration that occurs with aging is the primary risk factor for major human pathologies, including cancer, diabetes, cardiovascular disorders, and neurodegenerative diseases.
#3 We need to understand the problem — how we age, in order to come up with a solution to slow it down, and live healthier, longer
So, we want to identify the mechanisms of aging, and how they relate to the diseases that kill us, and then do something about it.
We cannot at this point stop aging, but we can slow it down. And for people who have been aging too quickly because of various lifestyle factors, such as a poor diet or lack of exercise — for those people, aging can be reversed in terms of improving the biomarkers associated with aging.
What I’m referring to here is a person’s biological age. Your biological age can be less than, equal to or greater than your calendar age. We notice this in the people we see every day, and we have a commonly expressed term for it, right? You look good for your age, or You’re fit for your age. Such comments are based upon some intuitive understanding of what a person of a certain chronological age looks like or functions.
I’m going to briefly describe each of these 14 Hallmarks of Aging, not because I’m sadistic and want to wear you down with the science, but because each one of these plays a role in how you age and in the diseases of aging.
And to some extent — for some Hallmarks more than others — these aging hallmarks have interventions; meaning, that there are things you can do to push back against many of the ways you age.
A Brief Depiction of the 14 Hallmarks of Aging
#1 Genomic instability
Genomic instability refers to the accumulation of DNA damage and mutations over time.
Your body’s cells are like tiny machines working diligently to keep everything running smoothly. These machines have an instruction manual of genetic instructions called DNA, which contains all the information needed to make cells function properly.
This is our genome. It’s the complete set of genes or genetic material present in a cell or organism.
But there’s a problem with our genome. As time goes by, these instruction manuals can become worn out and prone to errors. And that’s genomic instability.
#2 Telomere attrition
Shortening of protective caps at the ends of chromosomes can lead to genomic instability and cellular senescence.
Telomere attrition is the gradual shortening of protective caps at the ends of chromosomes. These caps are called “telomeres”. They naturally shorten with each cell division that occurs over time as we age.
When telomeres become critically short, cells can enter a state of senescence or apoptosis, which is another Hallmark of Aging that I’ll soon describe. (Apoptosis is a form of programmed cell death that occurs in multicellular organisms.)
#3 Epigenetic alterations
Changes in gene expression patterns due to modifications in DNA and histones can contribute to cellular dysfunction.
Epigenetic alterations are the changes in gene expression patterns due to modifications in DNA and histones. (Histones are like tiny spools that DNA wraps around to help keep genetic material organized. They play a role in controlling which genes are turned on or off.)
Such modifications can influence how genes are expressed or silenced, which strongly affects cellular function and overall health.
Relative to your healthy aging, this can work for you or against you. Without certain interventions, the age-related changes in these epigenetic modifications can disrupt normal cellular function and contribute to aging.
#4 Loss of proteostasis
Decline in the ability to maintain proper protein folding and clearance can lead to the accumulation of damaged proteins, contributing to cellular dysfunction.
Loss of proteostasis is the decline in the body’s ability to maintain proper protein folding, trafficking, and degradation within cells.
With age, protein maintenance becomes less efficient, leading to the accumulation of misfolded or damaged proteins, which contributes to cellular dysfunction, such as the creation of protein aggregates, which are basically the clumping together of proteins that can disrupt cellular functions, impair tissue health, and contribute to age-related diseases.
#5 Deregulated nutrient sensing
Dysregulation of nutrient-sensing pathways, such as insulin and mTOR signaling, can contribute to metabolic dysfunction.
Deregulated nutrient sensing involves the impairment of cellular mechanisms responsible for sensing and responding to nutrient availability in the body. This can affect cellular metabolism and promote aging.
Nutrient sensing pathways, such as insulin and mTOR, are responsible for detecting and responding to the availability of nutrients in our body, such as glucose, amino acids, and fatty acids. These pathways play a crucial role in coordinating cellular metabolism, growth, and energy utilization.
#6 Mitochondrial dysfunction
Impairment of energy production and increased production of reactive oxygen species can contribute to cellular dysfunction.
Mitochondrial dysfunction is the impairment of the body’s energy production. It leads to reduced energy production and an increased production of reactive oxygen species, which is a fancy word for free radicals. (Reactive oxygen species (ROS) describes a number of reactive molecules and free radicals derived from molecular oxygen.)
Mitochondria are organelles found in most human cells. They have their own DNA, apart from the cells’ DNA, and are responsible for producing energy for our body by converting chemical energy from food into a form that cells can use, such as ATP (adenosine triphosphate).
Without the ATP created by mitochondria, we would be dead within seconds.
Unfortunately, as we age, mitochondria become less efficient and functional. This can happen from a combination of genetic, environmental and oxidative factors. (Oxidative factors refer to conditions that may lead to oxidative stress, including obesity, poor diet, smoking, drinking alcohol, taking certain medicines, and exposure to environmental factors such as radiation, toxins, air pollution, pesticides, etc.)
Mitochondrial dysfunction leads to reduced energy production and increased production of harmful reactive oxygen species (ROS).
#7 Cellular senescence
Permanent cell cycle arrest and altered secretory phenotype can contribute to chronic inflammation and the aging process.
Cellular senescence occurs when specific cells stop growing — they become non-dividing — and enter a kind of “retirement” mode that they can’t get out of. These retired cells also start releasing signals called SASP that cause inflammation. (SASP is a pro-inflammatory phenotype known as the senescence-associated secretory phenotype.) As we get older, the number of these retired, inflammatory-producing cells increases. This causes problems in our tissues and makes us more prone to diseases related to aging.
#8 Stem cell exhaustion
Decline in the regenerative capacity of stem cells can contribute to tissue degeneration.
Stem cells have the ability to regenerate and repair tissues throughout our lifespan. But, as stem cells divide and differentiate, stem cell exhaustion sets in; meaning that their regenerative capacity decreases, and this leads to less tissue repair and regeneration.
#9 Altered intercellular communication
Disruption of signaling pathways between cells can contribute to chronic inflammation.
Cells communicate through various signaling pathways to ensure that our cells and organs work together in harmony. This includes signaling for hormones, neurotransmitters, immune cytokines, protein-based growth factors and insulin. (Immune cytokines are tiny protein that helps immune cells communicate with each other.)
As we get older, the signaling between cells can become impaired, become dysregulated, and this leads to compromised tissue homeostasis and functionality.
As we age, the way cells talk to each other can start to go haywire. This can mess up the balance and normal functioning of our tissues (referred to as tissue homeostasis).
#10 Disabled/Compromised macroautophagy
The cell’s self-cleaning process is compromised and not working properly.
Autophagy is a cellular process that removes damaged or dysfunctional components within the cell. This makes the cell healthier. As aging progresses, autophagy becomes less efficient, leading to the accumulation of cellular waste and unhealthy cells.
#11 Chronic/Systemic inflammation
Persistent low-grade inflammation can contribute to tissue degeneration.
Chronic inflammation is associated with most age-related diseases, and that’s why you’ll sometimes see it spelled inflammaging.
As we get older, our immune system can become overactive, causing low-level, but persistent inflammation. It’s like a smoldering fire that never quite goes out.
This ongoing inflammation can impact our health, making us more vulnerable to various diseases and conditions associated with aging.
#12 Microbiome disturbance/Dysbiosis
Imbalance in the composition of the microbiota can contribute to chronic inflammation
The microbiome refers to the trillions of microorganisms that reside in our bodies, particularly in the gut. Age-related changes in the microbiome composition can upset the balance between good and bad microbes, leading to inflammation, and problems in our gut and overall health.
#13 Altered mechanical properties
Changes in the physical strength and flexibility of cells and tissues associated with the aging process.
Aging affects the mechanical properties of tissues and organs, leading to stiffness and reduced elasticity. Regular physical activity and exercise can help maintain and improve tissue flexibility and strength
#14 Dysregulation of RNA processing/Splicing deregulation
The gerontologists at the 2020 the Copenhagen meeting identified this 14th Hallmark of Aging in the description of their five new hallmarks as Dysregulation of RNA Processing, but in their diagram they used a different term called “splicing deregulation” that speaks to slightly similar biological mechanism.
These two things are different enough to spend a moment distinguishing them from one another, so bear with me here, as I try to explain the difference between these two.
Dysregulation of RNA processing
Dysregulation of RNA processing in aging is like having a mix-up in the instructions that cells use to make proteins, leading to potential confusion and mistakes. Basically, with dysregulation, there’s a problem with the way our cells handle a vital molecule called RNA.
RNA is like a messenger that carries important instructions from our DNA to the cell’s machinery, telling it what to do and how to make essential proteins. In normal circumstances, this process is well-organized and precise, like a well-oiled machine.
However, when there’s dysregulation, it’s like the messenger is getting mixed up or making mistakes. It can send the wrong instructions or mess up the timing, which can lead to a variety of problems in the cell’s activities. These problems can contribute to various diseases, including some types of cancer and neurological disorders, because the cell isn’t functioning correctly due to these RNA processing errors. So, dysregulation of RNA processing is a key factor in many diseases where the cell’s communication system goes haywire.
Splicing deregulation
On the other hand, splicing deregulation is specifically about errors in the process of cutting and pasting genetic information to create functional proteins, which can also go awry as we get older. Both problems can affect how cells work and contribute to aging.
RNA splicing deregulation is specifically about errors in the process of cutting and pasting genetic information to create functional proteins, which can also go awry as we get older.
Think of RNA processing dysregulation like a complex orchestra performance where various instruments are playing out of sync or missing cues, affecting the overall harmony of the music.
Splicing deregulation, on the other hand, is like a jigsaw puzzle where the pieces don’t fit together properly, leading to a picture that doesn’t quite make sense.
While both involve disruptions in how things come together, one deals with the broader performance and the other with the precise arrangement of individual pieces.
The Hallmarks Are Interconnected
All of these aging hallmarks are interconnected cellular and molecular processes — each affecting the other — that contribute to the aging process and the development of age-related diseases.
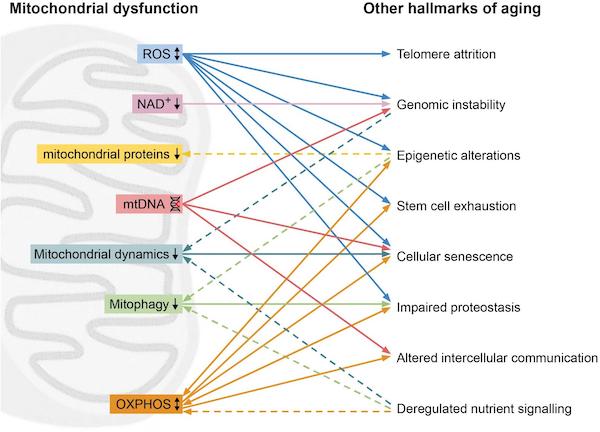
The illustration below presents an example of what I’m talking about; it depicts the interconnection between mitochondrial dysfunction evaluated by a study published in 2020:
Integrating the Hallmarks of Aging Throughout the Tree of Life: A Focus on Mitochondrial Dysfunction.
Mitochondrial dysfunction and Its Relation with Other Hallmarks
The above illustration shows how interconnected mitochondria are to the other Hallmarks of Aging, in this case the original nine, but the same is true for all the new Hallmarks of Aging as well.
Click here for a description of the illustration
Mitochondrial dysfunction (left) and its relation with other hallmarks (right). High levels of reactive oxygen species (ROS, blue) can lead to telomere attrition, genomic instability, epigenetic alternations, stem cell exhaustion, cellular senescence. On the other hand, ROS can also improve proteostasis. Low levels of NAD+ (pink) can lead to genomic instability. Epigenetic alterations can lead to reduced mitochondrial proteins (yellow) such as STEAP2, RHOT2, and RAB32. Mitochondrial DNA (mtDNA) damage (red) can result in genomic instability, cellular senescence and altered intercellular communication. Hallmarks of aging genomic instability and deregulated nutrient sensing can lead to reduced mitochondrial dynamics (turquoise), while reduced mitochondrial dynamics induces cellular senescence. Reduced mitophagy (green) leads to impaired proteostasis, while epigenetic alterations and deregulated nutrient sensing induce reduced mitophagy. Oxidative phosphorylation (OXPHOS, orange) affects the hallmarks epigenetic alterations, stem cell exhaustion, cellular senescence, impaired proteostasis, altered intercellular communication and is in turn affected by deregulated nutrient sensing, epigenetic alterations and impaired proteostasis. Dashed line represents an effect of a hallmarks of aging on the mitochondrial dysfunction.
You could think of this as a domino effect – when one domino falls, it knocks over the next one, and then the next, until the whole sequence is set in motion. But this is not always linear or strictly causal. Rather, there is a dynamic interplay between the hallmarks of aging and cellular signaling pathways that contribute to the development and progression of age-related diseases. (See Part 2.)
But, how does one Hallmark know about what’s happening with another? What is this “dynamic interplay” between them?
They know what’s happening because, in effect, cells talk to each other via many cellular signaling pathways. To understand that better and how this all contributes to the diseases that such the life out of us as we get older, we need to know the language of cells, the topic of Part 2.
OK, we’re done with describing the aging hallmarks. The stage is set for Part 2: How Disrupted Cellular Signaling Ages You which I’ll publish next week.
See you then.
Go to Part 2
Last Updated on April 16, 2024 by Joe Garma