My Review of Outlive by Dr. Peter Attia, Part 1

My review of Outlive by longevity expert, Dr. Peter Attia has just been released. This is Part 1, where I cover the first seven chapters that center on metabolic syndrome, perhaps the instigator of the Four Horsemen of the diseases that kill us. If you want to live long and healthy, you need these insights.
My review of Outlive: The Science and Art of Longevity, by Dr. Peter Attia and his co-author Bill Gifford (whose book Spring Chicken I reviewed here) has been six years in the making and very worth the wait. His co-author is Bill Gifford,
If you haven’t heard of Dr. Attia, it’s time to get introduced, and reading Outlive is a great way to do just
that, and to benefit from this man’s decades-long study of longevity through the experiences garnered by interviews of hundreds of experts, his personal adherence to best-practices, and his practice of longevity medicine at Attia Medical.
My review of Outlive will be delivered in three parts, this being Part 1. My aim is to give you enough of the book’s contents to whet your appetite to buy the book, the best small investment you can make to help make you live longer, better.
Dr. Attia begins the book by explaining what he refers to as Medicine 1.0, 2.0 and 3.0:
- Medicine 1.0 was the first era of medicine that lasted about two thousand years, exemplified by Hippocrates. Its conclusions were based on direct observation and guesswork.
- Medicine 2.0 is what’s mostly practiced now, and is mainly about dealing with the symptoms of disease.
- Medicine 3.0 is what Attia practices and advocates, but is rare. It deals with prevention by first understanding the seminal causes of the most pernicious and life-threatening chronic diseases that kill us, especially as we age.
This Part 1 review of Outlive covers the first eight chapters of his 17 chapter book. It deals with the prevalence of metabolic syndrome, one of the major killing diseases that he euphemistically refers to as the “Horsemen”, a reference to the four diseases most likely to kill you: heart disease, cancer, neurodegenerative disease and type 2 diabetes and related metabolic dysfunction.
To achieve longevity—to live longer and live better for longer—we must understand and confront these causes of slow death.
So, let’s begin.
How We Die in the Modern Era — The 4 Horsemen
Attia cleverly appropriates the “Four Horsemen of the Apocalypse” of biblical fame to represent the four chronic diseases, one of each — at least — has a good chance of killing us:
- Heart disease (aka atherosclerosis)
- Type 2 Diabetes
- Cancer
- Neurodegenerative diseases (aka Alzheimer’s and Dementia)
These four diseases tend to kill us slowly, because Medicine 2.0 waits till we’re close to the crisis stage until it intervenes. Attia relates several stories that underscore this reality, including his own, where well before middle age, and just after he swam the Catalina Channel in Southern California, the good doctor finds out that the roots of heart disease have firmly taken root.
To achieve longevity — to live longer and live better for longer — we must understand and confront these causes of slow death .
Living healthier, longer is to extend healthspan, and to do that necessitates delaying death from all four of the Horsemen. This is within the span of your control, but it requires consistent effort, for The Horsemen have one persistently powerful risk factor in common — age. As you grow older, the risk grows exponentially that one or more of the four diseases represented by the Horsemen has taken root in your body where it will steadily grow.
Of the four, heart disease is the most common instigator of death.
Heart disease and stroke (or cerebrovascular disease), which Attia lump together under the single heading of atherosclerotic cardiovascular disease, or ASCVD, is the leading cause of death, killing in 2020 an estimated 697,000 people in the United States; 1 in every 5 deaths (more than any other cause , including cancer), and 17.9 million people per year globally, accounting for at least 30% of all deaths worldwide.
Medicine 2.0 does not slow down the Four Horseman, and that’s the fundamental problem that Attia has with this “classic” practice of medicine. Using ASCVD as an example, the guidelines for managing cardiovascular risk are based on an overly short time horizon, compared to the timeline of the disease. It needs to be identified, prevented and treated much earlier than is typically done within the realm of Medicine 2.0.
Quoting professor Jay Olshansky of the University of Illinois at Chicago School of Public Health, Attia writes:
“We’re trying to attack heart disease, cancer, stroke, and Alzheimer’s one disease at a time, as if somehow these diseases are all unrelated to each other, when in fact the underlying risk factor for almost everything that goes wrong with us as we grow older, both in terms of diseases we experience, and of the frailty and disability associated with it, is related to the underlying biological process of aging.”
We need another approach, says Attia, and that’s the purview of Medicine 3.0.
Medicine 3.0 is characterized by a more personalized and proactive approach to healthcare. It emphasizes the use of advanced technology and data analysis to predict and prevent diseases before they occur. Medicine 3.0 also embraces the importance of lifestyle factors such as nutrition, exercise, and stress management in maintaining good, life-long health.
The 4 Horseman Ride the Insulin Path
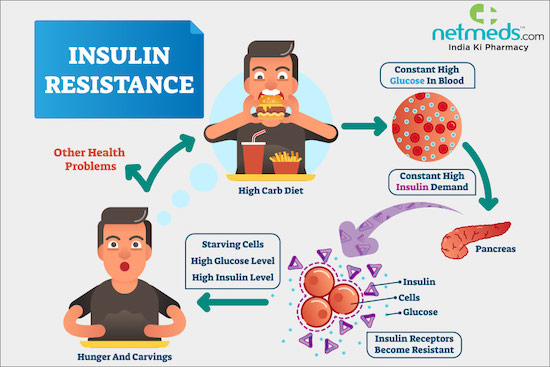
There’s a global epidemic of metabolic disorders that range from insulin resistance to type 2 diabetes. Insulin helps blood sugar enter the body’s cells so it can be used for energy, and signals the liver to store blood sugar for later use. When you eat food, your body converts that food into dietary sugars. Insulin is a hormone released by the pancreas that tells your cells to open up to that sugar and convert it into energy.
Insulin resistance is when cells in your muscles, fat and liver don’t respond well to insulin and can’t easily take up glucose from your blood. With insulin resistance, the cells don’t react, and don’t open up, resulting in excessive sugar in the blood. Over time, the pancreas keeps trying to regulate the blood sugar, producing more and more insulin until it wears out and can’t produce large amounts of insulin anymore. As a result, blood sugar levels increase to the point of being in the diabetic range.
Click here to watch Dr. Eleanna De Filippis, M.D., Ph.D., an endocrinologist at Mayo Clinic, explain insulin resistance.
Insulin resistance can lead to type 2 diabetes, but actually type 2 diabetes is a distinct disease clearly defined by various glucose metrics. Attia considers type 2 diabetes as “the last stop on a railway line passing through several other stations , including hyperinsulinemia, prediabetes, and NAFLD/NASH.” (NAFLD is nonalcoholic fatty liver disease, a condition in which excess fat builds up in your liver; and NASH, nonalcoholic steatohepatitis, is the form of NAFLD in which you have inflammation of the liver and liver damage, in addition to fat in your liver.)
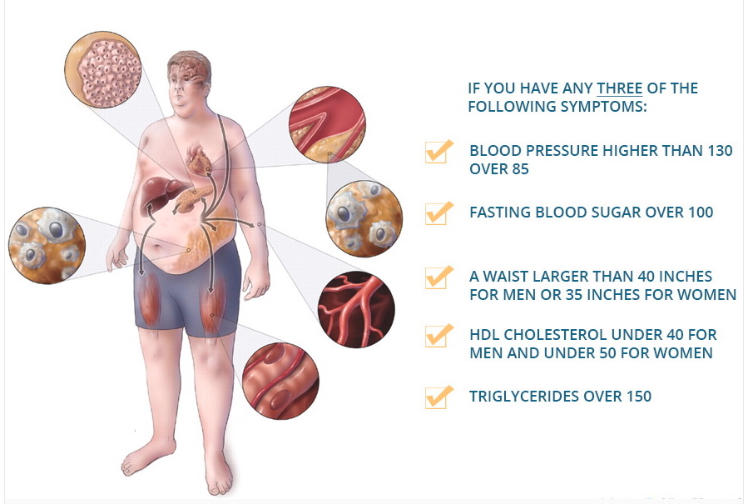
Once your body is resistant to insulin, you’re on the path to metabolic syndrome, which is defined as having three of the following five risk factors:
Attia reports that according to a 2020 article in JAMA (I don’t have the reference), about 90% of the U.S. population has at least one risk factor for metabolic syndrome, and “that nearly half of the population is either on the path to type 2 diabetes or already there.” As I wrote in Metabolic Syndrome Affects 30% of the U.S. Population — How About You?, about 30% of the U.S. population have three of the five risk factors that make them metabolically dysfunctional.
This concept of metabolic syndrome is valued by Attia, he says, because it helps identify these disorders as part of a continuum, as opposed to a single, binary condition. Medicine 2.0 routinely tests for these risk factors, if warranted, but is slow to respond until a person is well on his or her way of having three risk factors that indicate metabolic syndrome, or all five.
“Why wait until someone has three of the five markers?”, Attia asks, given that any one of them means that you’re sliding down a slippery slope to further health problems. A Medicine 3.0 approach would check for the warning signs years before there was a problem with the intention of intervening before a patient actually develops metabolic syndrome.
Attia lists a slew of biomarkers his clinic monitors related to metabolism, but the first thing he says he looks for, unsurprisingly, is elevated insulin. This for him is the “canary in the coal mine” of metabolic disorder, which is also ”associated with huge increases in one’s risk of cancer (up to twelvefold ), Alzheimer’s disease (fivefold ), and death from cardiovascular disease (almost sixfold) — all of which underscores why addressing, and ideally preventing, metabolic dysfunction is a cornerstone of my approach to longevity.”
Because people with diabetes have a much greater risk of cardiovascular disease, cancer, Alzheimer’s disease and other dementias, Attia argues that diabetes with related metabolic dysfunction is one thing that all these conditions have in common:
“This is why I place such emphasis on metabolic health, and why I have long been concerned about the epidemic of metabolic disease not only in the United States but around the world.”
Other metabolic-related topics discussed in Outlive
- Why humans have a unique capacity for turning calories from fructose into fat.
- Why fructose (ordinary sugar too) is a dastardly metabolic villain that even messes with the body’s production of ATP — cellular energy.
- Why you need to know about purines in certain meats, cheeses, anchovies, beer, etc., as well as uric acid (think gout).
- Why caloric restriction is a proven way to improve healthspan, and is connected to various cellular pathways, such as mTOR, AMPK and FOXO3.
- How the drugs rapamycin and metformin affect cellular pathways.
- How autophagy (cellular cleansing) is triggered by fasting, exercise and rapamycin.
Now, let’s turn to cholesterol. There’s lots here that you — and perhaps your doctor — does not know.
The Cholesterol Story that Matters
The cholesterol story is complicated, mainly because people haven’t kept up with the science about it, and because the only real important bits of it are rarely measured and considered.
First, it’s important to know that cholesterol is essential to life, given that it’s needed to produce some of the most important structures in the body, including:
- Cell membranes.
- Hormones such as testosterone, progesterone, estrogen and cortisol.
- Bile acids, which are necessary for digesting food.
Although all cells can synthesize their own cholesterol 20% of the supply made by our body is found in the liver, which you can visualize as a cholesterol repository of sorts that ships the cholesterol out to cells that need it and receives it back via blood circulation.
LDLs (low density lipoproteins) carry more lipids (think fats and oils), while HDLs (high density lipoproteins) carry more protein in relation to fat, and are therefore more dense.
Typically, when you go for your annual check up, your doctor orders a lipid panel that tests for LDL, HDL, total cholesterol and triglycerides (a type of lipid in blood). Once the results are in, you’ll be told that any of these measures are high, low or just right — but none of them really matter, because, as Attia dissects with precision, “it’s not the cholesterol per se that causes problems but the nature of the particle in which it’s transported.”
And that brings us to apoB.
Given the prevalence of heart disease — remember, it’s the world’s #1 killer — you must get your apoB tested, as I did a few months ago and was shocked by the result! This is because ApoB tests the aggregate of the LDL particles that cause atherosclerosis, and can lead to unhappy events like heart attacks.
Every single lipoprotein that contributes to atherosclerosis carries this apoB protein signature.
ApoB consists of:
- LDL – low density lipoprotein transports cholesterol from the liver to cells. 80% of its total weight contains lipids.
- VLDL – very low density lipoprotein transport triglycerides from the liver to adipose tissue. 91% of its total weight contains lipids.
- IDL – intermediate density lipoprotein are not usually detectable in the blood. Its size is between VLDL and LDL. 91% of its total weight contains lipids.
- LP(a) – Lipoprotein little a, an LDL with another special lipoprotein wrapped around it called apolipoprotein(a). This fella is the worst of all — it has an outsize ability to cause damage, even in relatively small numbers.
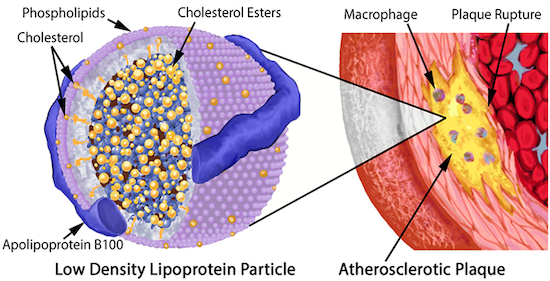
LDL particles become a problem when they begin to stick in the arterial wall and then become oxidized. This means the cholesterol (and phospholipid) molecules they contain come into contact with a highly reactive molecule known as a reactive oxygen species (ROS), the cause of oxidative stress. It’s the oxidation of the lipids on the LDL that kicks off the entire atherosclerotic cascade.
To discern your risk profile for atherosclerosis, the number of apoB particles circulating in your bloodstream must be determined. That number is much more relevant than the total quantity of cholesterol that these particles are carrying, not that the typical doctor practicing Medicine 2.0 will tell you that.
Medicine 2.0 has three blind spots when it comes to dealing with atherosclerotic disease, says Attia:
- First, it maintains an overly simplistic view of lipids that fails to evaluate the importance of total lipoprotein burden (apoB), and the necessity of minimizing it in order to truly reduce atherosclerotic risk.
- Second, a general lack of knowledge about apoB constituents, such as Lp(a).
- Third, a failure to fully grasp the lengthy time frame that encapsulates atherosclerotic disease progression, and the implications this carries if we seek true prevention.
Dr. Attia:
“ApoB not only tells me the concentration of LDL particles (which is more predictive of disease than the concentration of cholesterol found within LDL particles, LDL – C ), but it also captures the concentration of VLDL particles, which as members of the apoB family can also contribute to atherosclerosis. Furthermore , even someone whose apoB is low can still have a dangerously elevated Lp(a).”
So, you might wonder, how does all this cholesterol stuff actually cause atherosclerosis — a disease of the arteries characterized by the deposition of plaques of fatty material on their inner walls?
How atherosclerosis happens
Dr. Attia has vivid and detailed description of this processes that I’ll convey, inartfully, with a number of bullet points that provide the highlights:
- Aggregated or oxidized LDL on the artery wall instigates an immune response whereby macrophages (“big eater”) swallows it, but if it consumes too much of this cholesterol, they blow up into “foam cells”, so named because they look foamy under a microscope.
- When enough foam cells get together, they form a “fatty streak”, which is a precursor of an atherosclerotic plaque. (Teenagers typically already have some of these in their arteries.)
- The increasing number of foam cells begin to sort of ooze together into a mass of lipids, and become the core of our atherosclerotic plaque. In an attempt to control the damage, the “smooth muscle” cells in the artery wall migrate to this oozy waste site and secrete a kind of matrix in an attempt to build a kind of barrier around it, much like a scar.
- This matrix ends up as a new arterial plaque.
What can you do to slow down atherosclerotic progression?
You know the drill — get plenty of exercise, sleep, reduce stress, eat more plant food and much less processed meats and processed foods in general. But there’s two particular interventions that Attia explores that I want to spend a little time explaining:
- Reducing saturated fat, and
- Drugs.
Saturated Fat
Eating lots of saturated fat can increase levels of atherosclerosis – causing lipoproteins in blood, but Attia asserts that “most of the actual cholesterol that we consume in our food ends up being excreted out our backsides”, an assertion that Michael Greger, MD FACLM of Nutritionfacts.org contradicts.
In Attia’s clinical experience, about a third to half of people who consume high amounts of saturated fats (which sometimes goes hand in hand with a ketogenic diet) will experience a dramatic increase in the much unwanted apoB particles.
The reason for this is that (a) it appears that saturated fat contributes directly to the synthesis of excess cholesterol, and, more importantly, (b) excess saturated fat causes the liver to reduce expression of LDL receptors, thereby reducing the amount of LDL removed from circulation.
What we want is to increase apoB clearance by enhancing the body’s ability to get apoBs out of circulation. In large part, this is done by amplifying the activity of LDL receptors in the liver, which absorb cholesterol from the bloodstream.
I think this is sufficient reason to read food labels to see how much saturated fat is contained in the foods you eat, and to reduce your consumption of meat, if that’s something you frequently eat. (Typically meat is high in saturated fat).
Drugs
As mentioned, Dr. Attia devoted himself to lowering his chance of getting some form of atherosclerosis after he learned that he was primed for it. He learned that he has a genetic propensity for heart disease, underscored by the number of deaths by heart attack in his family tree. For him, lifestyle interventions were necessary but insufficient, and so like so many millions of people, he needed a statin.
Statins inhibit cholesterol synthesis, prompting the liver to increase the expression of LDL receptors, resulting in more LDL taken out of circulation. They may have other benefits too , including an apparent anti-inflammatory effect.
People who have a high apoB score (like I do) might need a PCSK9 inhibitor and/or a statin. Subscribers to my Newsletter will be briefed once I learn if some interventions I tried brought down my apoB level.
I learned about my apparent apoB problem because I’m devoted to getting the type of care that Medicine 3.0 offers, which includes following cutting-edge experts in the medical field, such as Dr. Attia. Because if you want a longer, healthier life, you need to take a much longer view, and seek “to identify and eliminate the primary causative agent in the disease process…”
In the case of apoB, Attia says:
“Once you understand that apoB particles — LDL , VLDL , Lp(a) — are causally linked to ASCVD [atherosclerotic cardiovascular disease], the game completely changes. The only way to stop the disease is to remove the cause, and the best time to do that is now.”
Other cholesterol-related topics discussed in Outlive
- Do you need to be concerned about apoA, as well as apoB?
- What actually makes HDL particles potentially “ good ”
- Why it’s hard to detect atherosclerotic progression with normal techniques, and what to do now.
Cancer and Metabolism
Cancer is considered the second Horseman, and presents a greater challenge in treatment compared to heart disease, the first Horseman. Medicine 3 advocates for more frequent testing, particularly for cancers with accurate screening methods such as lung, prostate, breast, and colon cancer. Early detection is crucial, and for certain cancers like colon cancer, testing should begin a decade earlier, at 40 years old instead of 50.
While interventions for non-metastasized cancers, such as surgery, can be effective, treating metastasized cancers proves difficult. The good news here is that promising modalities like immunotherapy, which aims to mobilize the immune system to identify and destroy cancer cells, and cancer metabolism, are making headway.
Cancer cells primarily produce ATP anaerobically, a less efficient method than the aerobic metabolism used by normal cells. ATP (adenosine triphosphate) is a molecule that serves as the primary energy carrier in cells. It plays a central role in cellular metabolism, which encompasses all the chemical processes required to sustain life. Through the lens of cancer metabolism, the connection between ATP and metabolism is particularly significant.
In normal cells, energy production primarily occurs through aerobic metabolism, a highly efficient process that involves the breakdown of glucose in the presence of oxygen to produce ATP. This process generates a large amount of energy for the cell’s needs while also producing carbon dioxide and water as byproducts.
In contrast, cancer cells often exhibit altered metabolism, favoring anaerobic metabolism, also known as the Warburg effect. This process involves the breakdown of glucose without the need for oxygen, leading to the production of ATP and a byproduct called lactic acid. The reliance on anaerobic metabolism is less efficient than aerobic metabolism, resulting in cancer cells consuming much higher levels of glucose to sustain their energy demands.
The connection between ATP and cancer metabolism becomes critical in understanding cancer growth and proliferation. Cancer cells’ preference for anaerobic metabolism allows them to thrive even in oxygen-deprived environments within tumors. This metabolic shift helps fuel the uncontrolled growth and division of cancer cells, contributing to tumor progression.
Researchers are exploring how to leverage cancer metabolism against the disease. As cancer cells consume significantly more glucose than regular cells, one approach involves combining targeted chemotherapy with caloric restriction (CR) and/or a ketogenic diet, which is very low-carb (glucose). These interventions aim to starve cancer cells of glucose, which they require to survive and proliferate.
In 2011, Douglas Hanahan and Robert Weinberg, two prominent cancer researchers, identified two key hallmarks of cancer that have since driven new treatments and strategies for reducing cancer risk. The first hallmark involves the altered metabolism of cancer cells, leading to their increased consumption of glucose. The second hallmark pertains to cancer cells’ ability to evade the immune system’s surveillance and destruction mechanisms, a challenge that researchers like Steve Rosenberg have tirelessly sought to address for many years. These hallmarks offer promising avenues for developing more effective treatments and preventative measures against cancer.
Neurodegenerative Diseases (Alzhimer’s)
Alzheimer’s disease stands as the final challenge, the last of the Horsemen we must overcome on our journey to reach the centenarian milestone, writes Attia. Unfortunately, Medicine 2.0 proves inadequate in this battle, as it often waits until symptoms are pronounced before intervening, weakening the impact of preventative measures.
The key to the best prevention lies in obtaining an early assessment. While you or a family member might notice certain indicators, such as issues with verbal fluency and memory, associative memory, spatial memory, or hearing loss, these signs are not conclusive. However, if these indicators persist, they should serve as sufficient reason to seek help from a neurologist who can perform thorough tests to aid in diagnosis. Dr. Attia, for instance, has a preventive neurologist on his staff, Dr. Kellyann Niotis (see him interview her below).
The concept of preventing Alzheimer’s disease gained scientific support when a two-year randomized controlled trial in Finland showed that interventions involving nutrition, physical activity, and cognitive training helped maintain cognitive function and prevent decline in at-risk older adults. Encouraging results from other large European trials with lifestyle-based interventions also offered hope.
Notably, women face a higher age-adjusted risk of Alzheimer’s and experience faster disease progression overall, irrespective of age and education. Although the fact that more women live to age eighty-five and above partly explains this discrepancy, there seems to be something unique about menopause that dramatically increases the risk of neurodegeneration in older women. Specifically, a rapid decline in estradiol levels in women with an e4 allele appears to be a driver of risk. This observation suggests the potential role of perimenopausal hormone replacement therapy in these women.
In the following video, Dr. Attia interviews Dr. Kellyann Niotis is a neurologist specializing in risk reduction strategies for the prevention or slowing of neurodegenerative disorders.
Topics covered:
- An overview of the various diseases associated with neurodegeneration, including, but not limited to, Alzheimer’s disease, Lewy body dementia, and Parkinson’s disease.
- An in-depth explanation of Parkinson’s disease, including its pathology, role in movement capacity, very early warning signs, and the role of anxiety and sleep.
- An in-depth discussion of Alzheimer’s disease, including the latest in screening, genetics, and tools/strategies for prevention.
- An explanation about the differences and commonalities among the various diseases of neurodegeneration and the potential causative triggers.
- The importance of early screening, cognitive testing, and taking the proper steps to lowering the risk of disease.
Click her for topics time stamp
0:00:00 – Intro
0:00:08 – Kellyann’s background, training, and interest in the brain
:03:33 – A primer on neurodegeneration: different types, prevalences, interventions, and more
0:14:44 – Overview of Parkinson’s disease and neuromuscular disorders including ALS 0:16:26 – Parkinson’s disease: early signs, diagnosis, genetics, causative triggers, and more
0:31:12 – Interventions to delay or avoid Parkinson’s disease, and the role of sleep and anxiety
0:40:26 – The challenge of standardizing early interventions for Parkinson’s disease without a clear biomarker
0:49:22 – Alzheimer’s disease: pathophysiology and the role of the amyloid and tau proteins
0:53:16 – Can PET scans be informative for diagnosing Alzheimer’s disease?
0:59:28 – Tau accumulation in the brain, tau scans, serum biomarkers, and possible early detection of Alzheimer’s disease pathology
1:06:43 – Cognitive testing explained
1:18:55 – The challenge of identifying the stage of the disease and why drugs have not shown efficacy 1:22:23 – The association between hearing loss and dementia
1:26:39 – The relationship between oral health and neurodegenerative diseases
1:29:58 – Genetic risk for Alzheimer’s disease
1:39:17 – What one’s mitochondrial haplotype can reveal about their risk of neurodegenerative disease 1:43:23 – The positive impact of exercise on brain health
1:46:34 – High blood pressure as a risk factor
1:51:22 – Why women are disproportionately affected by Alzheimer’s disease
1:54:14 – Final takeaways: the future of understanding neurodegenerative disease and further reducing risk
My Review of Outlive, Part 2
In the upcoming Part 2 of my review of Outlive, I will cover several more chapters that address cancer, Alzheimer’s and other neurodegenerative diseases, and exercise.
Here, I tried to impress upon you Attia’s exhortation that:
“It is beyond backwards that we do not treat hyperinsulinemia [excess insulin in the blood] like a bona fide endocrine disorder of its own. I would argue that doing so might have a greater impact on human health and longevity than any other target of therapy.”
As concerns cancer, Alzheimer’s and other neurodegenerative diseases, you’ll learn that they are aggravated in some way by metabolic dysfunction; as such, there will be no doubt in your mind that the “logical first step in our quest to delay death is to get our metabolic house in order.”
As I mentioned earlier, and what Attia affirms, is that improving our metabolic status is within the realm of our control:
“Changing how we exercise, what we eat , and how we sleep can completely turn the tables in our favor”.
That’s the good news.
The bad news is that none of those good things happen unless you commit yourself to the effort:
“… these things require effort to escape the default modern environment that has conspired against our ancient ( and formerly helpful) fat-storing genes, by overfeeding, undermoving, and undersleeping us all.
Here’s My review of Outlive, Part 2
Subscribe to my Newsletter!
Last Updated on July 22, 2023 by Joe Garma