Slow Down Biological Aging with Dr. Matt Kaeberlein

To slow down biological aging is the dream of millions of people who recognize that they can influence how they age. Let renowned longevity scientist, Dr. Matt Kaeberlein guide you. (Watch)
The latest info on how to slow down biological aging is presented in a three-hour interview with Dr. Matt Kaeberlein. You can:
- Watch Simon Hill interview Kaeberlein with very insightful and pertinent questions about the biology aging and how to slow it down, or
- Read my summary of the interview, or
- Do both.
Whatever you do, at least get a sense of what Dr. Kaeberlein knows about aging, do you can do your best to slow it down and optimize your healthspan.
What follows is the video from the Proof Podcast, hosted by Simon Hill, and then my summary organized by the same chapter titles and time stamps provided by Mr. Hill.
Check out Simon Hill’s channel.
let’s dig into the summary…
Aging and Disease (03:42)
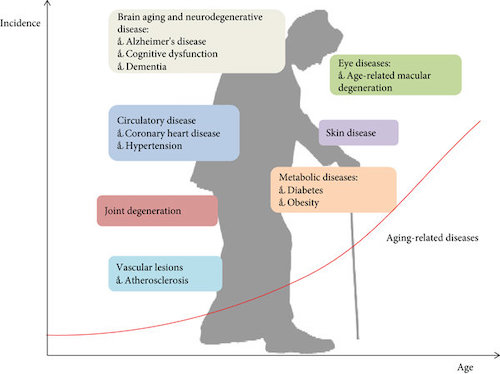
Dr. Kaeberlein discusses the relationship between aging and disease, explaining that aging is the largest risk factor for many age-related diseases. He discusses how aging is a complex and multifactorial process that involves a combination of genetic, environmental and lifestyle factors — each of which needs to be considered to slow down biological aging.
Important points
Biological aging is the root cause of most of the major diseases that cause death and disability in developed countries. If we can modify biological aging, then we can have an impact on all of these different functional declines and diseases that go along with old age.
The biology of aging refers to what’s happening on the cellular, molecular, tissue and organ level of an organism. As these things become compromised, biological aging occurs.
The mechanisms that link aging biology and disease processes are going to be somewhat different for the different diseases. For cardiovascular disease there are going to be functional changes within the heart itself that are different from what occurs relative to biological aging; for instance, aging can lead to mitochondrial dysfunction that contributes to declines in heart function that lead to decreases in circulatory capacity.. There are also systemic effects that are not unique to the heart, such as inflammatory signals given off by senescent cells that contribute to vascular dysfunction and the dysfunction of other tissues and organs.
Some background info
Mitochondrial dysfunction is one of the original nine Hallmarks of Disease that I cover here. For more on senescent cells, read my post, Can 4 Natural Compounds “Eliminate Senescent Cells” and Prolong Your Healthy LIfespan?
Evolutionary Conserved Processes and Human Lifespan (17:41)
Dr. Kaeberlein explains that there are evolutionary conserved processes that impact human lifespan and healthspan, such as the mTOR pathway and the insulin/IGF-1 pathway. He discusses how interventions that target these pathways, such as calorie restriction and rapamycin, have been shown to extend lifespan and improve healthspan in model organisms.
Important points
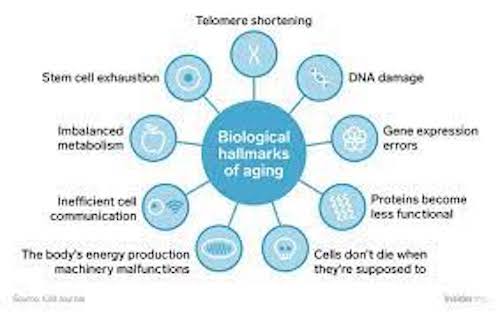
The Hallmarks of Aging are thought to explain the biology of aging in all organisms evaluated, from nematode worms to fruit flies to mice to rats to dogs and people. This speaks to the principle of evolutionary conservation, which in this case means that the mechanisms of biological aging are common to all organisms. This is one reason why it’s helpful to study various aging processes and interventions in various life forms. To some extent, the findings of such studies in other animals could be relevant to humans.
Environment plays a huge role in the rate of biological aging. By environment, Kaeberlein is not only speaking to things like the quality of air and water, but also various lifestyle factors such as diet, exercise and sleep.
Metabolism plays a huge role in biological aging. Referring back to the Hallmarks, Kaeberlein describes them as a network of information that decays over time and thereby drives biological aging, particularly metabolism.
There appears to be a direct relationship between metabolism and lifespan in various organisms, referred to as the Rate of Living theory. It postulates that metabolic rate is a primary determinant of a species lifespan. Referring back to his network idea, Kaeberlein posits that another determinant of a species lifespan is probably also impacted by how well equipped the species is to deal with the breakdowns that go along with the aging of his “information network. An organism will live longer and in better health should it possess functional repair mechanisms that maintain homeostasis (maintaining resilience and balance). Those that have better repair capacity are going to age more slowly and be able to handle the breakdown of the system and last longer.
Many experiments have found that aging can be manipulated. The largest lifespan extensions studied thus far come from restricting calories. This has been true and everything from worms up through monkeys, and is thought to be also true for humans.
The second largest impact on extending longevity comes from a drug called rapamycin.
Approximately 75% of human longevity is influenced by lifestyle factors, and 25% is controlled by genetics. One exception to that is if a person has a variant for a disease allele that might kill a person prematurely. (See background information.)
Some background info
An allele is one of two or more alternative forms of a gene that arise by mutation and are found at the same place on a chromosome. The most common allele of concern is also the most potentially damaging, and that concerns Alzheimer’s.
People who inherit one copy of the APOE e4 allele have an increased chance of developing the disease; those who inherit two copies of the allele are at even greater risk. It is important to note that people with the APOE e4 allele inherit an increased risk of developing Alzheimer disease, not the disease itself. (More here.)
Biological Aging: Are Epigenetic Tests More Accurate in Telling How Well You’re Aging? (28:09)
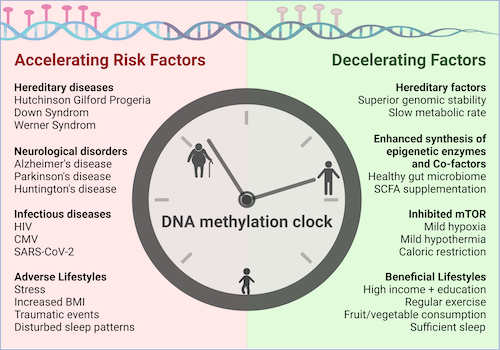
 Dr. Kaeberlein discusses the use of epigenetic testing to measure biological aging and how it may be a more accurate measure of an individual’s aging than chronological age. He explains how different epigenetic markers can be used to measure different aspects of aging, such as DNA methylation and histone modifications.
Dr. Kaeberlein discusses the use of epigenetic testing to measure biological aging and how it may be a more accurate measure of an individual’s aging than chronological age. He explains how different epigenetic markers can be used to measure different aspects of aging, such as DNA methylation and histone modifications.
Important points
Epigenetic variation is one of the Hallmarks of Aging. The genome is all the genetic information of an organism, and the epigenome is a record of the chemical changes to the DNA and histone proteins of an organism. Your genome is fixed, but your epigenome is affected by lifestyle factors, which have an outsized role in determining the genes that are expressed (turned on or off).
Epigenetic tests aimed at measuring biological age are typically focused on measuring epigenetic changes, something that Kaeberlein believes is an oversimplification of the complexity of biological aging. Although he thinks epigenetic age can be measured with some precision, it’s more important to know the functional measures of aging and hormonal changes that accompany aging. For instance, traditional blood tests are more informative than epigenetic age tests, because we already know what such tests mean and what the interventions might be based upon test results. Functional measures are clearly useful, such as measuring how fast you can run, or how long you can run, or how much weight you can lift, or various mobility and stability capabilities. Such tests clearly convey relevant information about your health. But regarding epigenetic tests, he says that we don’t really know what the epigenetic age tests mean and how they might be actionable.
Some background
For a deeper dive into epigenetics, read my three-part series that covers the topic and the various tests that use epigenetic markers to estimate biological age.
Aging Process and the Burden of Senescent Cells (34:06)
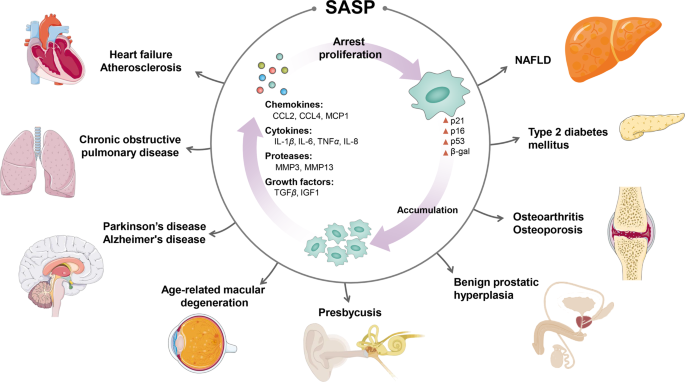
Senescence-associated secretory phenotype (SASP) is a phenotype associated with senescent cells wherein those cells secrete high levels of inflammatory cytokines, immune modulators, growth factors, and proteases.
Dr. Kaeberlein discusses the burden of senescent cells, which are cells that have stopped dividing and become dysfunctional. He explains how the accumulation of senescent cells can contribute to age-related diseases and how interventions that target these cells, such as senolytics, have been shown to improve healthspan.
Important points
The Hallmarks of Aging provide an important common basis from which to examine the mechanisms of aging, but be aware that the relative importance of each of those Hallmarks in different cell types is different. Nonetheless, one commonality between all cell types is that as cells divide, they accumulate various types of damage, including DNA damage, that leads to less functional mitochondria and shorter telomeres, among other things, such as the transformation from normal cells to senescent cells. When this happens they switch to a proinflammatory state, whereby they emit inflammatory cytokines that contribute to much of the inflammation that becomes prevalent as we get older.
There is a domino effect with senescent cells, because the inflammatory cytokines that they give off affect nearby healthy cells, making them senescent, which then affect nearby cells making them senescent, on it goes. As we get older, this burden of increasing numbers of senescent cells negatively impacts stem cells, making them stop functioning the way they’re supposed to, thereby contributing to a decline in tissue health that we observe with aging, as well as an increased risk of cancer.
Some background
Mitochondria are organelles inside most cells, almost like a cell within a cell, that has it’s own DNA. Their job is to make ATP, the energy the cells use to exist. Cytokines are any of a number of substances, such as interferon, interleukin, and growth factors, which are secreted by certain cells of the immune system and have an effect on other cells.
Keys to a Longer, Healthier Life (45:08)
Dr. Kaeberlein provides practical tips for living a longer, healthier life, such as maintaining a healthy diet, engaging in regular exercise, getting adequate sleep, managing stress, and maintaining social connections. He also discusses the potential benefits of fasting and calorie restriction for extending healthspan.
Important points
Kaeberlein doesn’t get explicit in identifying any specifics about the important lifestyle factors he mentions, such as sleep, exercise and diet, although he does encourage the consumption of fermented foods.
He does underscore the importance of sleep, that it’s fundamentally tied into the biology of aging, but offers no specifics until the next section topic below, Nature and Aging.
What he does address most fully in terms of lifestyle factors is the importance of minimizing stress. People who are subject to high levels of anxiety, stress and depression tend to have shorter telomeres which is associated with a fast rate of aging, or a higher biological age relative to their chronological age.
Kaeberlein expresses his focus on happiness as one keystone to healthy aging. He says that it’s important to avoid social isolation and to cultivate a sense of community.
Some background
For more on telomeres and stress, read my post, How Depression and Stress Makes Us Age Faster.
Sleep and Exercise Slow Down Biological Aging (55:58)
Dr. Kaeberlein discusses the importance of sleep and exercise for healthy aging, explaining how sleep deprivation and lack of exercise can contribute to age-related diseases. He also provides practical tips for improving sleep and incorporating exercise into daily routines.
Important points
Poor sleep quality is strongly associated with a higher likelihood of cognitive dysfunction and dementia. Deep sleep enables the clearing of damage from the brain, but unfortunately the quality of sleep declines with age. Unfortunately, the mechanite mechanisms behind this are not clearly understood.
Alcoholic consumption “trashes your sleep quality”, and in this case he’s made a concerted effort to cut way back on the amount of alcohol he consumes, particularly prior to bed.
Regarding exercise, Kaeberlein says that he’s unaware of studies showing that consistent exercise increases lifespan, but the data is clear that it improves healthspan tremendously, those years over which you’re healthy. Exercise improves mitochondrial function, one of the Hallmarks of Aging, and he thinks there is good evidence that consistent exercise reduces inflammation one of the prominent drivers of aging, and that it has the ability to up regulate autophagy, which is important for cleaning aggregated proteins, another homework of aging.
What is particularly interesting is how he addresses the seeming paradox about the role of mTOR and aging. mTOR is a nutrient signaling pathway that is associated with many aspects of aging, which you’ll read more about below.
The paradox is that upleveling mTOR is necessary for maintaining or improving muscle mass — a necessary condition for healthy aging — but at the same time numerous studies from many different labs show that inhibiting mTOR is associated with increased lifespan and healthspan in every animal model studied. These studies, however, have been done independently from the context of muscle function. How to modulate between mTOR inhibition and expression is currently unclear.
mTOR oscillates throughout the day, going up and down, activated by amino acids (protein) consumption, and deactivated by a lack of protein and by fasting. The expression of mTOR in different tissues also varies. Given this complexity, it remains unclear exactly how to finesse the upregulation of mTOR in muscle tissues when you want that to happen, say during and after exercise, and its downregulation the rest of the time.
Rapamycin is currently the most effective mediator of mTOR, and given that you might think that it would also affect the muscle-centric benefits of exercise, but that does not appear to be the case. Studies show that rapamycin consumption does not impair muscle function.
Kaeberlein believes that although mTOR is activated while in muscle activation mode, It’s probably not being activated in heart, brain, liver and other tissues. Likewise, when rapamycin is taken, mTOR is probably being transiently inhibited in most tissues, perhaps all tissues to some degree, but with different effects in different tissues. He underscores that, at least in mice, rapamycin protects against the age-related loss of muscle, sarcopenia, a common feature of aging. It’s been shown that in mice and dogs, rapamycin is protective against sarcopenia, but we don’t know if that’s true for humans.
He concludes this section by underscoring the importance of sleep and exercise. He says that it’s very important to maintain an active lifestyle by finding activities that you enjoy and do over the long term. He focuses on resistance training, which he does three or four times a week. His training is mostly done with compound exercises (exercises that require multiple muscles), such as the squat, deadlift, bench press and pull up. He also does cardio three or four times a week, which includes hiking the hills around Seattle where he lives, or inside the gym on an elliptical machine.
Some background
Mammalian target of rapamycin (mTOR) regulates cell proliferation, autophagy, and apoptosis by participating in multiple signaling pathways in the body. Studies have shown that the mTOR signaling pathway is also associated with cancer, arthritis, insulin resistance, osteoporosis, and other diseases.
I’ve written a lot about exercise and sleep.
CR and Prolonged Fasting Slow Down Biological Aging (1:15:23)
Dr. Kaeberlein discusses the potential benefits of calorie restriction and prolonged fasting for extending healthspan, explaining how these interventions can improve metabolic health and cellular function.
Important points
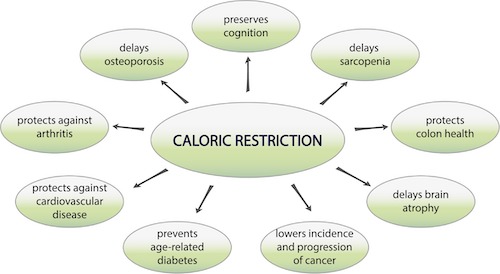
Caloric Restriction is the largest single intervention effect on lifespan that’s been found. Many studies show that caloric restriction can significantly increase lifespan in all animal models reviewed. In mice and rats with certain genetic backgrounds, lifespan can be increased as high as 50 or 60%.
This effect seems to be linear in mice:
- A 20% restriction of calories yields about a 20% increase in lifespan.
- A 50% restriction on calories yields about a 50% increasing lifespan.
Kaeberlein says that combining the caloric restriction with an intermittent feeding schedule provides a greater lifespan extension than just reducing calories, but allowing the animal study to eat whenever they want (ad libitum). The restricted calories are the key, not when they eat; for instance, if you reduce the time allotted for feeding but those animals eat the same amount of calories as another group with no time restriction, then the lifespan effect is insignificant.
Despite the assertion of the paragraph above, recent studies are trying to incorporate the effect of circadian rhythms, meaning to have the feeding window be concurrent with the time of day that the animals are fed combined with the effects of caloric restriction. More data is needed from such studies, but Kaeberlein is confident that the circadian clock matters, such that if, say, a mouse is calorically restricted but is able to eat at a time inconsistent with its circadian clock (remember they are nocturnal), then the benefits of caloric restriction tend to be nullified.
He emphasizes that once a normal weight level is achieved you want to maintain whatever calories you were consuming to bring you to that weight level. As an example, he says that if your goal is 135 pounds, you will want to restrict calories, say by 500 calories per day if that’s sustainable, until you get 135 pounds, and then eat sufficient calories to maintain that weight.
Regarding fasting, or fasting mimicking diets, Kaeberlein says that there are lots of evidence that this can be a pretty potent anti-inflammatory effect and can boost autophagy, whereby you can restore mitochondrial function, but you need to be careful with how long you fast because you don’t want to lose too much muscle tissue or complicate any chronic issues that you might have.
Some background
A “fasting mimicking diet” (FMD) consists of a precise selection of macro and micronutrients that do not activate cellular nutrient signaling pathways, such as mTOR, similar to what occurs during fasting. The most renown FMD is ProLon. I’ve done this program, as has my mother, and chronicled our experiences with video in the post, The ProLon FMD — Get Better, Not Older.
Protein Restriction and Its Implications (1:32:14)
Dr. Kaeberlein discusses the potential benefits of protein restriction for extending healthspan, explaining how high protein intake can activate the mTOR pathway and contribute to age-related diseases.
Important points
Kaeberlein echoes what anyone who has read the various studies, or listened to the various pundits about protein quickly discover — there’s lots of contradictory information about how much protein to consume and from what sources.
One of the problems is trying to disentangle protein consumption from overall caloric consumption. Some studies show lifespan benefits or healthspan benefits from reducing protein, but does that reflect a reduction of total calories overall, which is known to confer such benefits, or is it something about that particular macronutrient, protein?
He thinks that it’s true that a high protein diet combined with high fat and high sugar would probably increase the risk of cancer, or your risk of dying prematurely overall, but Kaeberlein does not see the evidence that higher protein by itself is detrimental, unless consumed in extreme amounts, or if a person has kidney disease.
He says that the recommended daily allowance (RDA) in the United States for protein is 0.8 grams per kilogram of body weight was developed to see what sedentary men would need to prevent nitrogen imbalance, which could lead to muscle loss. But this amount is not ideal. He asserts that more than 0.8 is better. In his case, he aims for 1.6 grams/kilo, an amount he deems sufficient to fuel muscle repair and growth given his resistance training.
Kaeberlein does acknowledge that highly respected scientists such as Dr Valter longo has done compelling research that indicates that the 0.8 number is generally a good protein consumption level to maintain, or slightly more for people 60 years or older, given that the ability to utilize dietary protein through a process called protein muscle synthesis declines as we get older, and therefore there’s a need to consume more protein in order to get the same effect as did a lower amount consumed when younger.
Dr Longo has studied how protein intake affects IGF-1 levels, because high protein consumption increases IGF1, which if sustained can lead to an increase in all-cause mortality. Kaeberlein points out, however, that studies have also shown that those who perform resistance training along with their higher protein diet do not experience the same increase in IGF1 as groups who ate the same amount of protein but did not do resistance training.
Some background
Read my post, 8 Sure-fire Ways to Trim Body Fat and Keep It Off Forever: The Best Quality Protein.
Plant Protein vs Animal Protein (1:43:50)
Dr. Kaeberlein discusses the potential differences between plant protein and animal protein in terms of their impact on health and aging.
Important points made
He supports the generally accepted notion that animal sources of protein do offer a better muscle protein synthesis then plant-based protein; nonetheless, he tries to get about 70% of his protein from plant sources and 30% from high quality meat sources.
Those who advocate for lower protein consumption often site the example of the people who live on the Japanese island of Okinawa who tend to have a low protein consumption. Kimberlin makes a point, however, that overall okinawan’s eat approximately 20% less calories than mainly in Japanese, so it’s unclear if they’re remarkable longevity results from an overall caloric restriction versus a lower amount of a particular macronutrient, in this case protein.
Some background
For more about protein sources, read my post, Plant Protein vs Animal Protein.
Kaeberlein’s proportions of animal vs plant protein sources nicely squares with the protein consumption of many of the Blue Zoners, as I detail in the post, The Blue Zone Diet Will Transform You.
Natural Occurring Polyphenols and Supplements Related to Longevity (1:53:26)
Dr. Kaeberlein discusses the potential benefits of natural occurring polyphenols and supplements for extending healthspan, explaining how these compounds can improve cellular function and reduce inflammation.
Important points made
Although Kaeberlein advocates the consumption of plant foods given that they’re rich in fiber and polyphenols, he says that it’s hard to get definitive information about the mechanistic action of polyphenols on health outcomes because they are so-called “dirty” molecules That means that polyphenols, such as resveratrol from red wine and a few other sources, have many downstream effects, so it’s difficult to isolate just one.
Regarding resveratrol, Kaeberlein admits his bias against its usefulness. He spent a lot of time and effort trying to reproduce the results from prior research extolling the virtues of resveratrol, but was unable to generate similar data. That said, he agrees that resveratrol has biological activity that targets many different proteins in the cell, but any derivative benefits are unclear to him, he says.
The original paper that sparked the interest in resveratrol was done in yeast ,and it reported a 70% increase in lifespan extension, but Kaeberlein asserts that this didn’t really happen. The other problem with resveratrol he says is that it does not actually activate the so-called longevity genes, “sirtuins”.
There is much debate as to whether sirtuins are longevity genes, and if they’re conserved, meaning that most organisms exhibit longevity benefits from their activation. Kaeberlein says that scientists argue about longevity benefits derived by activating sirtuins in humans (which resveratrol was said to do).
Regarding various supplements that are portrayed to be beneficial for extending healthspan/lifespan in humans, he is skeptical. He says that it’s important for those in the field of gerontology to say unequivocally that from a pharmaceutical or supplement perspective there’s nothing currently that is going to modulate the biology of aging in a way that’s going to increase healthspan or lifespan in humans, even though in the laboratory, this has been done in some animals such as a mice.
Regarding the popular NAD precursors NMN and NR, Kaeberlein says some initial studies claimed lifestyle extension in certain animals, but they were not reproduced elsewhere.
Kaeberlein also briefly touches on other popular so-called anti-aging supplements, like alpha-ketoglutarate that did seem to attenuate the frailty in mice and marginally increase their lifespan, as well as spermidine, which has been reported to increase lifespan in a couple of different studies in mice. He says that if further studies validate their potential for improving health span or a lifespan, he might consider taking NAD precursors as well as alpha-ketoglutarate.
Regarding NAD itself, Kaeberlein says that there is evidence that NAD declines with age, at least in some tissues, and that that plays into the Hallmark of Aging model, particularly in regard to metabolic dysfunction, mitochondrial dysfunction and DNA damage. Thus, there’s some evidence supporting a causal role here, but he’s unsure if it’s definitive and is looking for more evidence to support the idea that NAD dysregulation could play a role in the biology of aging.
You would think, he says that if any NAD was a big contributor to lifespan extension, you could treat, or upregulate it with precursors, such as NMN and NR, and restore the decline and achieve a meaningful lifespan improvement, but to date that hasn’t been shown.
Regarding which NAD precursor, NMN or NR is better, he says his intuition is that you can raise intracellular NAD with both, and he thinks of them as being roughly equivalent.
Some background info
Here are some of my posts relevant to this topic:
Rapamycin and Derivatives Slow Down Biological Aging (2:14:14)
Dr. Kaeberlein explains how rapamycin is a compound that has been shown to extend lifespan in mice and may have similar effects in humans. He discusses the potential downsides, including negative effects on the immune system and the fact that it is a prescription drug. He also talks about rapamycin derivatives, which may have similar effects on aging without the negative side effects.
Important points
Rapamycin has been mentioned several times already, but in this section the interviewer, Simon Hill, and Kaeberlein hone in on this molecule.
If you haven’t heard about rapamycin, it’s a small molecule that was first found on Easter Island off the coast of Chile, which is also referred to as Rapa Nui. Rapamycin is produced by a bacteria from the soil from Rapa Nui. It was initially studied for its ability to prevent cell division with antifungal properties. Eventually, it was approved by the FDA to prevent organ transplant rejection because of its immunomodulatory effects; in other words, an immunosuppressant that now has been used for more than 20 years.
In the early 2000s, four different labs independently found that if you could turn down mTOR, you could increase lifespan in three different organisms: yeast, the nematode worm and in fruit flies. The drug that could do this was rapamycin. Scientists really got excited about this in 2009, says Kaeberleinin, when the National Institute on Aging Interventions Testing Program showed that you can increase lifespan in mice with rapamycin. Remarkably, lifespan extension in mice via rapamycin could happen even when the treatment started at 20 months of age, equivalent to a 60 year-old human. This was the first time that anybody showed that an intervention this late in life could be started and still get big benefits.
Subsequent studies showed that you could reverse or delay aging in nearly every tissue and organ of a mouse with rapamycin. To delay aging at this point was not uncommon, as the aforementioned caloric restriction studies showed, but to actually reverse financial declines in some tissues and organs was startling.
One of Kaeberlein’s major recent areas of study is in an on-going, long-term, double blind placebo controlled clinical trial in pet dogs using rapamycin to answer the question, Does rapamycin increase lifespan and healthspan in pet dogs?
Some background info
Many scientists who are aware of rapamycin or study it, including Kaeberlein himself, take rapamycin, as due thousands of biohackers, but there’s a risk because there is no clinical evidence about the efficacious dose. And as the saying goes, the dose is the poison, which refers to the fact that something can either be beneficial or harmful depending on the dose.
Rapamycin doses taken are all over the map, but Kaeberlein says that 6 milligrams administered once a week, by a syringe, is what is commonly used with few negative effects. I don’t want to say more about this, because I don’t want to encourage you to take rapamycin without medical oversight. Although at a low dosage the only commonly reported side effect is mouth sores, there is the potential for a higher risk of infection given that rapamycin is an immunosuppressant. It can also increase triglycerides that could have cardiovascular consequences longer term, as well as dysregulate of glucose homeostasis.
Anti-aging Compounds Slow Down Biological Aging? (2:30:09)
Dr. Kaeberlein discusses other compounds that may have anti-aging effects, again referring to NAD precursors, which can increase levels of NAD in the body and potentially have positive effects on aging-related processes. He also discusses metformin, a drug commonly used to treat type 2 diabetes, which has been shown to have some anti-aging effects.
Important points
Kaeberlein had touched on some of these potential age arresting compounds before, but here the focus was mainly on metformin.
Metformin is a safe drug that has been used over several decades in type 2 diabetics. It was discovered that diabetics on the metformin were living longer than healthy non-diabetics. This observations instigated a lot of interest and much further research.
With metformin the relevant comparisons should compare diabetics not taking metformin, diabetics taking metformin and non-diabetics not taking metformin. What you want to see is if in these three different populations there is a difference in mortality or life expectancy.
The general consensus is that diabetics taking metformin live longer than non-diabetics taking metformin, but there were diversions in the case of diabetics taking metformin compared to non-diabetics not taking metformin. In this regard, there were two contradicting studies, one of which showed a small benefit from metformin in terms of improving aging in humans, whereas the other study suggested a detriment to metformin.
So from a purely perspective of lifespan enhancement, the jury is still out on metformin. A few things that are widely understood is that metformin can have a significant decline in testosterone, free testosterone levels, which can hamper the quality of life, especially in men. Metformin may also impair some of the benefits of exercise, which I cover here.
Kaeberlein doesn’t think it makes much sense for people who are not glucose impaired (type 2 diabetics, or pre-diabetics), to take metformin given what’s currently known.
Some background info
You might be interested in the following posts:
And that’s it. Scroll below to the Comments section should you wish to ask a question or leave a comment. If you want to be alerted when I write a new post, become a Subscriber:
Subscribe to my Newsletter!
Last Updated on September 21, 2023 by Joe Garma